Abstract
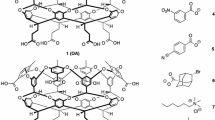
Resorc[4]arene based peptide-cavitands with four identical chiral amino acids at their upper rim were synthesized and investigated for the complexation of small guest molecules. Competition experiments show, that the tetra amino acid cavitands complex small organic guests in chloroform in the order ethyl acetate <dichloromethane < acetonitrile < ethanol < acetamide < acetic acid. The peptide-cavitands containing aspartic and glutamic acid derivatives enclose parts of their attached amino acids in the cavity, so that these peptide-cavitands are host and guest at the same time. The starting material for the cavitands, the resorc[4]arenetetraamine 3, is made by a new synthetic route using the Delépine-reaction.








Similar content being viewed by others
References
(a) Moran, J.R., Ericson, J.L., Dalcanale, E., Bryant, J.A., Knobler, C.B., Cram, D.J.: Vases and kites as cavitands. J. Am. Chem. Soc. 113, 5707 (1991); (b) Cram, D.J., Karbach, S., Kim, H.-E., Knobler, C.B., Maverick, E.F., Ericson, J.L., Helgeson, R.: Host-guest complexation. 46. Cavitands as open molecular vessels form solvates. J. Am. Chem. Soc. 110, 2229 (1988); Tunstad, L.M., Tucker, J.A., Dalcanale, E., Weiser, J., Bryant, J.A., Sherman, J.C., Helgeson, R.C., Knobler, C.B., Cram, D.J.: Host-guest complexation. 48. Building blocks for cavitands and carcerands. J. Org. Chem. 54, 1305 (1989)
(a) Timmerman, P., Verboom, W., Reinhoudt, D.N.: Resorcinarenes. Tetrahedron 52, 2663 (1996); (b) Jasat, A., Sherman, J.C.: Carceplexes and hemicarceplexes. Chem. Rev. 99, 931 (1999); (c) Verboom, W.: Cavitands. In: Asfari, Z., Böhmer, V., Harrowfield, J.McB., Vicens, J. (eds.), Calixarenes 2001, Kluwer, Dordrecht (2001)
(a) Boerrigter, H., Grave, L., Nissink, J.W.M., Chrisstoffels, L.A.J., van der Maas, J.H., Verboom, W., de Jong, F., Reinhoudt, D.N.: (Thio)urea resorcinarene cavitands. Complexation and membrane transport of halide anions. J. Org. Chem. 63, 4174 (1998); (b) Ahn, D.-R., Kim, T.W., Hong, J.-L.: Water-soluble resorcin[4]arene: Complexation of anionic aromatic Guests by cooperativity of electrostatic and hydrophobic interactions. Tetrahedron Lett. 40, 6045 (1999)
(a) Mezo, A.R., Sherman, J.C.: Cavitands are effective templates for inducing stability and native like structure in De Novo four-helix bundles. J. Am. Chem. Soc. 121, 8983 (1999); (b) Gibb, B.C., Mezo, A.R., Causton, A.S., Fraser, J.R., Tsai, F.C.S., Sherman, J.C.: Efficient coupling of amino acid derivatives to rigid organic scaffolds: Model syntheses for De Novo proteins. Tetrahedron 51, 8719 (1995)
(a) Hamuro, Y., Calama, M.C., Park, H.S., Hamilton, A.D.: Ein Calixaren mit vier Peptidschleifen: ein Antikörper-Mimeticum zur Erkennung von Proteinoberflächen. Angew. Chem. 109, 2797 (1997); (b) Hamuro, Y., Calama, M.C., Park, H.S., Hamilton, A.D.: A calixarene with four peptide loops: An antibody mimic for recognition of protein surfaces. Angew. Chem. Int. Ed. Engl. 36, 2680 (1997); (c) Lin, A., Hamilton, A.D.: Design and synthesis of multiple-loop receptors based on a calix[4]arene scaffold for protein surface recognition. Comptes Rendus 5, 441 (2002); (d) Sansone, F., Barboso, S., Casnati, A.,Fabbi, M., Pochini, A., Ugozzoli, F., Ungaro, R.: Synthesis and Structure of Chiral Cone Calix[4]arenas Functionalized at the Upper Rim with L-Alanine Units. Eur. J. Org. Chem. 897 (1998); (e) Bauer, W., Soi, A., Hirsch, A.: Application of DPFGSE-ROE to calixarene derivatives under conditions near NOE zero-crossing. Magn. Reson. Chem. 38, 500 (2000); (f) Lazzarotto, M., Sansone, F., Baldini, L., Casnati, A., Cozzini, P., Ungaro, R.: Synthesis and properties of upper rim C-linked peptidocalix[4]arenes. Eur. J. Org. Chem. 595 (2001)
Berghaus, C., Feigel, M.: Peptide-Cavitands based on resorc[4]arenes – synthesis and structure. Eur. J. Org. Chem. 3200 (2003)
Boerrigter , H., Verboom, W., Reinhoudt, D.N.: Novel resorcinarene cavitand-based CMP(O) cation ligands: synthesis and extraction properties. J. Org. Chem. 62, 7148 (1997)
The reaction type is known as the Delépine reaction: Delépine, M.: Sur l´hexaméthyléne-amine (suite). Solubilités, hydrate, bromure, sulfate, phosphate. Bull. Soc. Chim. Fr. 13, 352 (1895)
Boerrigter, H., Verboom, W., Reinhoudt, D.N.: Ligands for EuIII, FeIII, SrII, and UO2II based on CMPO-functionalized resorcinarene cavitands; Synthesis and Extraction. Liebigs Ann./Recueil, 2247 (1997)
MacroModel V4.0: Mohamadi, F., Richards, N.G.J., Guida, W.C., Liskamp, R., Lipton, M., Caufield, C., Chang, G., Hendrickson, T., Still, W.C.: MacroModel – An Integrated Software System for Modeling Organic and Bioorganic Molecules Using Molecular Mechanics. J. Comput. Chem. 11, 440 (1990)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hülsbusch, C.M., Feigel, M. Complexing and self-complexing of peptide-cavitands. J Incl Phenom Macrocycl Chem 59, 53–63 (2007). https://doi.org/10.1007/s10847-007-9294-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-007-9294-3