Abstract
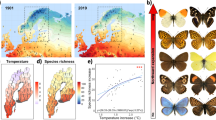
Climate change is currently considered one key threat to biodiversity. Species with a restricted distribution possibly will be more affected than those with wide ranges. Climate change can potentially affect both herbivores and their host plants and reduce their geographical ranges. The nature and intensity of their responses, however, may not necessarily match. We investigated the synergistic effects of climate change on two Neotropical butterfly species and their respective host plants at the end of twenty-first century. The species selected contrast in distribution extent, feeding habits and conservation status: Battus polystictus is widespread, oligophagous and common and Parides ascanius has a restricted distribution, is monophagous and is listed as vulnerable in the IUCN red list. Maps of the potential distribution of the butterflies and their host plants, as well as maps showing the changes in the ranges, in overlap area and direction of shifts were produced. Under forecasted climate change, all ranges and interaction areas decreased and the impacts were proportional to the intensity of change in future scenarios, either when compared all together or pairwise (p < 0.001). Based in our results estimation of climatically suitability, the monophagous butterfly with restricted distribution did suffer more severely these effects than the widespread generalist species. We did not anticipate, however, the possible strength of the predicted effects. Under the conditions modelled, P. ascanius would probably find no suitable conditions for occurrence, irrespectively of its host plant, and might go extinct. B. polystictus, on the other hand, suffered marked decreases in suitable area (46% for RCP4.5 and 91% for RCP8.5) and dramatic southward shifts (> 1439 km for RCP4.5 and > 1956 km for RCP8.5) on its range. This effect is further worsening because although most host plants are also much affected by the changes, the shift in their ranges is on average much smaller and each species responded in subtly different ways to the changing conditions, so that most of their future range may be spatially incompatible with the B. polystictus. We propose that the extinction risk of P. ascanius should be adjusted to critically endangered and point that species interactions and climate change must be accounted for in conservation planning.
Implications for insect conservation
The assessment carried out in this study contributes to the knowledge of climate change scenarios of butterfly species correlated with their host plants until the end of this century. These results can propose priority sites for conservation efforts like contribute to change status of P. ascanius to critically endangered, actually listed as vulnerable on the IUCN red list.




Similar content being viewed by others
References
Allouche O, Tsor A, Kadmon R (2006) Assessing the accuracy of species distribution models: prevalence, kappa and the true skill statistic (TSS). J Appl Ecol 43:1223–1232
Anderson RP, Martınez-Meyer E (2004) Modeling species’ geographic distributions for preliminary conservation assessments: an implementation with the spiny pocket mice (Heteromys) of Ecuador. Biol Conserv 116(2):167–179
Araújo MB, Luoto M (2007) The importance of biotic interactions for modelling species distributions under climate change. Glob Ecol Biogeogr 16:743–753. https://doi.org/10.1111/j.1466-8238.2007.00359.x
Araújo MB, Rozenfeld A (2014) The geographic scaling of biotic interactions. Ecography. https://doi.org/10.1111/j.1600-0587.2013.00643.x
Archibald SB, Johnson KR, Mathewes RW, Greenwood DR (2011) Intercontinental dispersal of giant thermophilic ants across the arctic during early eocene hyperthermals. Proc R Soc 278:3679–3686
Bale JS, Masters GJ, Hodkinson ID, Awmack C, Bezemer TM, Brown VK, Butterfield J, Buse A, Coulson JC, Farrar J, Good JE, Harrington R, Hartley S, Jones TH, Lindroth RL, Press MC, Symrnioudis I, Watt AD, Whittaker JB (2002) Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Glob Change Biol 8(1):1–6
Barve N, Barve V, Jiménez-Valverde A, Lira-Noriega A, Maher SP, Peterson AT, Soberón J, Villalobos F (2011) The crucial role of the accessible area in ecological niche modeling and species distribution modeling. Ecol Model 222:1810–1819
Bonebrake TC, Ponisio LC, Boggs EPR (2010) More than just indicators: a review of tropical butterfly ecology and conservation. Biol Conserv 143:1831–1841
Bonfantti D, Di Mare RA, Giovenardi R (2009) Butterflies (Lepidoptera: Papilionoidea and Hesperioidea) from two forest fragments in northen Rio Grande do Sul. Braz Check List 5(4):819–829
Boulangeat I, Gravel D, Thuiller W (2012) Accounting for dispersal and biotic interactions to disentangle the drivers of species distributions and their abundances. Ecol Lett 15:584–593. https://doi.org/10.1111/j.1461-0248.2012.01772.x
Brown KS, Freitas AVL (2000) Atlantic forest butterflies: indicators of landscape conservation. Biotropica 32(4b):934–956. https://doi.org/10.1111/j.1744-7429.2000.tb00631.x
Butterflies of America (2019) A website devoted to the study and enjoyment of American butterflies. https://www.butterfliesofamerica.com/. Accessed 30 June 2019
Callaghan CJ, Casgrande MM, Lamas G, Mielke OH, Pyrcz TW, Robbins RK, Viloria AL (2004) Atlas of neotropical Lepidoptera. Checklist: Part A 4 5:439
Condamine FL, Sperling FA, Wahlberg N, Rasplus J, Kergoat GJ (2012) What causes latitudinal gradients in species diversity? Evolutionary processes and ecological constraints on swallowtail biodiversity. Ecol Lett 15(3):267–277
Da Cunha HF, Ferreira ÉD, Tessarolo G, Nabout JC (2018) Host plant distributions and climate interact to affect the predicted geographic distribution of a Neotropical termite. Biotropica 50(4):625–632
DB-City (2018) A source of information on every country in the world. Société Advercity. https://pt.db-city.com. Accessed 12 June 2018
Diniz-Filho JA, Mauricio Bini L, Fernando Rangel T, Loyola RD, Hof C, Nogués-Bravo D, Araújo MB (2009) Partitioning and mapping uncertainties in ensembles of forecasts of species turnover under climate change. Ecography 32(6):897–906
Dirzo R, Young HS, Galetti M, Ceballos G, Isaac NJB, Collen B (2014) Defaunation in the anthropocene science defaunation anthropocene. Science 345(6195):401–406
Ehrlich PR, Raven PH (1964) Butterflies and plants: a study in coevolution. Evolution 18(4):586–608
Essens T, Van Langevelde F, Vos RA, Van Swaay CA, WallisDeVries MF (2017) Ecological determinants of butterfly vulnerability across the european continent. J Insect Conserv 21(3):439–450. https://doi.org/10.1007/s10841-017-9972-4
Eva HD, De Miranda EE, Di Bella CM, Sgrenzaroli OM, Jones S, Elvidge C (2002).A vegetation map of South America. European Commission
FAO - Food and Agriculture Organization of the United Nations (2018) The Food and Agriculture Organization. http://www.fao.org. Accessed 10 Mar 2018
Ferro V, Lemes P, Melo AS, Loyola R (2014) The reduces effectiveness of protected areas under climate change threatens atlantic forest tiger moths. PLoS ONE. https://doi.org/10.1371/journal.pone.0107792
Fielding AH, Bell FJ (1997) A review of methods for the measurement of prediction errors in conservation presence/absence models. Environ Conserv 24:38–49
Fox R, Oliver TH, Harrower C, Parsons MS, Thomas CD, Roy DB (2014) Long-term changes to the frequency of occurrence of British moths are consistent with opposing and synergistic effects of climate and land-use changes. J Appl Ecol 51(4):949–957
Freitas AVL, Leal JR, Uehara-Prado M, Iannuzzi L (2006) Insetos como indicadores de conservação da paisagem. Biol Da Conserv Essênc 1:357–385
Freitas AVL, Marini-Filho OJ, Mielke OHH, Casagrande MM, Brown KS Jr., Kaminski LA, Iserhard CA, Ribeiro DB, Dias FM, Dolibaina DR, Carneiro E, Uehara-Prado M, Romanowski HP, Emery EO, Accacio GM, Rosa AHB, Bizarro JMS, Silva ARM, Guimarães MP, Silva NAP, Braga L, Almeida G (2018) Parides ascanius (Cramer, 1775). In: instituto chico mendes de conservação da biodiversidade (ed) livro vermelho da fauna brasileira ameaçada de extinção. volume vii-invertebrados. ICMBio, Brasília, pp 93–95
Fuller T, Munguia M, Mayfield M, Sánchez-Cordero V, Sarkar S (2006) Incorporating connectivity into conservation planning: a multi-criteria case study from Central Mexico. Biol Conserv. https://doi.org/10.1016/j.biocon.2006.04.040
GBIF - Global Biodiversity Information Facility (2019) free and open access to biodiversity data. https://www.gbif.org/.m. Accessed 22 June 2019
Giannini TC, Tambosi LR, Acosta JR, Saraiva AM, Imperatriz-Fonseca VL, Metzger JP (2015) Safeguarding ecosystem services: a methodological framework to buffer the joint effect of habitat configuration and climate change. PLoS ONE 10(6):e0129225. https://doi.org/10.1371/journal.pone.0129225
Grice H, Freitas AVL, Rosa A, Marini-Filho O, Mega N, Silva F, Mielke O, Casagrande M (2018) Parides ascanius
Habibullah S, Din BH, Tan SH et al (2022) Impact of climate change on biodiversity loss: global evidence. Environ Sci Pollut Res 29:1073–1086. https://doi.org/10.1007/s11356-021-15702-8
Halsch CA, Shapiro AM, Thorne JH, Waetjen DP, Forister ML (2020) A winner in the anthropocene: changing host plant distribution explains geographical range expansion in the gulf fritillary butterfly. Ecol Entomol 45(3):652–662. https://doi.org/10.1111/een.12845
Hijmans RJ, Graham CH (2006) The ability of climate envelope models to predict the effect of climate change on species distributions. Glob Change Biol 12(12):2272–2281
Hijmans RJ, Phillips S, Leathwick J, Elith J (2013) Dismo: species distribution modelling. R package version 0.8-11
Hijmans RJ, Phillips SJ, Leathwick JR, Elith J (2016) dismo: species distribution modeling. R package ver. 1.0-15
Hill JK, Thomas CD, Fox R, Telfer MG, Willis SG, Ascher J, Huntley B (2002) Responses of butterflies to twentieth century climate warming: implications for future ranges. Proc R Soc Lond 269:2163–2171. https://doi.org/10.1098/rspb.2002.2134
Hoffmann D, Vasconcelos MF, Martins RP (2015) How climate change can affect the distribution range and conservation status of an endemic bird from the highlands of eastern brazil: the case of the gray-backed Tachuri, Polystictus superciliaris (Aves, Tyrannidae). Biota Neotrop 15(2):1–12. https://doi.org/10.1590/1676-060320150075
IPCC (2019) Summary for policymakers. In: Shukla PR, Skea J, Calvo Buendia E (eds) Climate change and land: an IPCC special report on climate change, desertification, land degradation, sustainable land management, food security, and greenhouse gas fluxes in terrestrial ecosystems
IUCN Standards and Petitions Committee (2019) Guidelines for using the IUCN red list categories and criteria. version 14. prepared by the standards and petitions committee. http://www.iucnredlist.org/documents/RedListGuidelines.pdf. Accessed 15 Jan 2020
Karatzoglou A, Smola A, Hornik K, Zeileis A (2004) kernlab-an S4 package for kernel methods in R. J Stat Softw 11(9):1–20
Koh LP, Sodhi NS, Brook BW (2004) Co-Extinctions of Tropical Butterflies and their Host plants. Biotropica 36(2):272–274
Lemes L, de Andrade AFA, Loyola R (2019) Spatial priorities for agricultural development in the Brazilian Cerrado: may economy and conservation coexist? Biodivers Conserv 29(5):1683–1700
Lewinsohn TM, Novotny V, Basset Y (2005) Insects on plants: diversity of herbivore assemblages revisited. Annu Rev Ecol Evol Syst 36:597–620. https://doi.org/10.1146/annurev.ecolsys.36.091704.175520
Lima VP, de Lima RAF, Joner F et al (2022) Climate change threatens native potential agroforestry plant species in Brazil. Sci Rep 12:2267. https://doi.org/10.1038/s41598-022-06234-3
Lima-Ribeiro MS, Varela S, González-Hernández J, Oliveira G, Diniz-Filho JA, Terribile LC (2015) EcoClimate:a database of climate data from multiple models for past, present, and future for macroecologists and biogeographers. Biodivers Inform 10:1–21
MapBiomas (2019) Projeto de Mapeamento Anual da Cobertura e Uso do Solo do Brasil. http://mapbiomas.org. Accessed 30 Aug 2019
Marengo JA, Chou S, Torres RR, Giarolla A, Alves LM, Lyra A (2014) Climate change in central and South America: recent trends, future projections, and impacts on regional agriculture
Medhaug I, Stolpe MB, Fischer EM, Knutti R (2017) Reconciling controversies about the ‘global warming hiatus.’ Nature 545:41–47. https://doi.org/10.1038/nature22315
Mega NO, Scalco VW, Atencio GWG, Morais ABB, Romanowski HP (2015) Battus polydamas (Lepidoptera: Papilionidae). Uses the Open-Field Aristolochia sessilifolia (Piperales: Aristolochiaceae) as its host plant in Uruguayan Savanna Areas. Fla Entomol 98:762–769. https://doi.org/10.1653/024.098.0255
Mesquita CP, King AJ, Schmidt SK, Farrer EC, Suding KN (2016) Incorporating biotic factors in species distribution modeling: are interactions with soil microbes important? Ecography. https://doi.org/10.1111/ecog.01797
Metzger JP, Bustamante MMC, Geraldo JF, Fernandes W, Librán-Embid F, Pillar VD, Prista PR, Rodrigues RR, Vieira ICG, Overbecki GE (2019) Why brazil needs its legal reserves. Perspect Ecol Conserv 17(3):104–116
Molina-Martínez A, Leon-Cortes JL, Regan HM, Lewis OT, Navarrete D, Caballero U, Luis-Martinez A (2016) Changes in butterfly distributions and species assemblages on a Neotropical Mountain range in response to global warming and anthropogenic land use. Divers Distrib 22(11):1085–1098. https://doi.org/10.1111/ddi.12473
Moss RH, Edmonds JA, Hibbard KA, Manning MR, Rose SK, Vuuren DPV, Carter TR, Emori S, Kainuma M, Kram T, Meehl GA, Mitchell JFB, Nakicenovic N, Riahi K, Smith SJ, Stouffer RJ, Thomson AM, Weyant JP, Wilbanks TJ (2010) The next generation of scenarios for climate change research and assessment. Nature 463:747–756
New TR, Pyle RM, Thomas JA, Thomas CD, Hammond PC (1995) Butterfly conservation management. Ann Rev Entomol 40:57–83
Otero LS, Brown KS (1984) Biology and ecology of Parides ascanius (Cramer, 1775) (Lep. Papilionidae), a primitive butterfly threatened with extinction. Atala 10:2–16
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst 37(1):637–669. https://doi.org/10.1146/annurev.ecolsys.37.091305.110100
Parmesan C, Yohe GA (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–43. https://doi.org/10.1038/nature01286
Parmesan C, Rttholm N, Stefanescu C, Hill JK, Thomas CD, Descimon H, Huntley B, Kaila L, Kullberg J, Tammaru T, Tennent WJ, Thomas JA, Warren M (1999) Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature 399:579–583. https://doi.org/10.1038/21181
Quintero I, Wiens JJ (2013) What determines the climatic niche width of species? the role of spatial and temporal climatic variation in three vertebrate clades. Glob Ecol Biogeogr 22(4):422–432
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.r-project.org/. Accessed 31 Mar 2018
Racheli T, Pariset L (1992) II genere Battus - Tassonomia e Storia Naturale
Rambo B (1956) The physiognomy of rio grande do sul: a natural monograph essay, vol 6. Selbach Bookstore
Revelle WR (2017) psych: procedures for personality and psychological research
Ribeiro MC, Metzger JP, Martensen AC, Ponzoni FJ, Hirota MM (2009) The brazilian atlantic forest: how much is left, and how is the remaining forest distributed? Implic Conserv Biol Conserv 142(6):1141–1153. https://doi.org/10.1016/j.biocon.2009.02.021
Robinet C, Roques A (2010) Direct impacts of recent climate warming on insect populations. Integr Zool 5(2):132–142. https://doi.org/10.1111/j.1749-4877.2010.00196.x
Sala OE, Chapin FS, Armesto JJ, Berlow E, Bloomfield J, Dirzo R, Huber-Sanwald E, Huenneke LF, Jackson RB, Kinzig A, Leemans R, Lodge DM, Mooney HA, Oesterheld M, Poff NL, SYKES MT, Walker BH, Walker M, Wall DH (2000) Global biodiversity scenarios for the year 2100. Science 287(5459):1770–1774
Scalco VW, Morais ABB, Romanowski HP, Mega NO (2015) Population dynamics of the swallowtail butterfly battus polystictus polystictus (Butler) (Lepidoptera: Papilionidae) with notes on Its natural history. Neotrop Entomol 45(1):33–43
Scarano FR (2019) Biodiversity sector: risks of temperature increase to biodiversity and ecosystems. In: Nobre C, Marengo J, Soares W (eds) climate change risks in Brazil. Springer, Cham, pp 131–141
Schweiger O, Settele J, Kudrna O, Klotz S, Kühn I (2008) Climate change can cause spatial mismatch of tropically interacting species. Ecology 89(12):3472–3479
Scriber JM (2010) Integrating ancient patterns and current dynamics of insect plant interactions: taxonomic and geographic variation in herbivore specialization. Insect Sci 17:471–507
Seraphim N, Barreto MA, Almeida GSS, Esperanço AP, Monteiro RF, Souza AP, Freitas AVL, Silva-Brandão KL (2016) Genetic diversity of Parides ascanius (Lepidoptera: Papilionidae: Troidini): implications for the conservation of Brazil’s most iconic endangered invertebrate species. Conserv Genet 17(3):553–546
Sobral-Souza T, Francini RB, Lima-Ribeiro MS (2015) Species extinction risk might increase out of reserves: allowances for conservation of threatened butterfly Actinote quadra (Lepidoptera: Nymphalidae) under global warming. Nat Conserv 13(2):159–165
Specieslink (2019) http://www.splink.org.br/. Accessed 20 June 2019
Tabari H, Willems P (2018) Seasonally varying footprint of climate change on precipitation in the Middle East Hossein. Sci Rep 8(1):1–10
Tyler H, Brown KS, Wilson K (1994) Swallowtail butterflies of the Americas—a study in biological dynamics, ecological diversity, biosystematics, and conservation (ISBN 0-945417-90-X)
Uehara-Prado M, Fonseca RL (2007) Urbanization and mismatch with protected areas place the conservation of a threatened species at risk. Biotropica 39(2):264–268
Vautard R, Gobiet A, Sobolowski S, Kjellström E, Stegehuis A, Watkiss P, Mendlik T, Landgren O, Nikulin G, Teichmann C, Jacob D (2014) The european climate under a 2 °C global warming. Environ Res Lett 9(3):034006
Wahlberg N, Leneveu J, Kodandaramaiah U, Peña C, Nylin S, Freitas AVL, Brower AVZ (2009) Nymphalid butterflies diversify following near demise at the Cretaceous/Tertiary boundary. Proc R Soc B 276:4295–4302
Walther GR, Post E, Convey P, Menzel A, Parmesan C, Beebee TJ et al (2002) Ecological responses to recent climate change. Nature 416(6879):389
Williams JW, Jackson ST (2007) Novel climates, no-analog communities, and ecological surprises. Front Ecol Environ 5:475–482
Winkler IS, Mitter C, Scheffer SJ (2009) Repeated climate-linked host shifts have promoted diversification in a temperate clade of leaf-mining flies. Proc Natl Acad Sci 106(43):18103–18108
Zanin M, Tessarolo G, Machado N, Albernaz ALM (2017) Climatically-mediated landcover change: impacts on brazilian territory. An Acad Bras Ciênc 89:939–952
Acknowledgements
We thank for the visited collections and curators that supported with this paper: Alexandre Soares, Renato de Oliveira E. Silva, Dr. Andy Warren. We thank as well for the support: Dr. Mirna Casagrande, Dr. Olaf Mielke, Dr. Lucas Kaminski, Dr. Marcelo Duarte, Dr. Guilherme Atencio, MSc. Carla Cenci, MSc. Diego Martins, Dr. Ricardo Siewert, Dr. Ana Paula Carvalho, Dr. Ryan St. Laurent, Dra. Sidia Maria Callegari Jacques. This study was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10841_2022_407_MOESM1_ESM.tif
Figure S1. Potential distribution maps for the host plants: a) Aristolochia arcuata, b) Aristolochia galeata, c) Aristolochia gigantea, d) Aristolochia macroura , e) Aristolochia melastoma, f) Aristolochia triangularis predicted by the The Ecological Niche Models (ENMs) derived from three coupled Atmosphere-Ocean General Circulation Models (AOGCM) in pres (present) and future - RCP4.5, optimistic scenario and RCP8.5, pessimistic scenario (TIF 100936 kb)
10841_2022_407_MOESM2_ESM.tif
Figure S2. Change in potential distribution of the Aristolochia host plants in the future (RCP4.5 – optimistic and RCP8.5 – pessimistic): a) Aristolochia arcuata, b) Aristolochia galeata, c) Aristolochia gigantea , d) Aristolochia macroura , e) Aristolochia melastoma, f) Aristolochia triangularis predicted by the The Ecological Niche Models (ENMs) derived from three coupled Atmosphere-Ocean General Circulation Models (AOGCM). Color indication: green: current and future stability; blue: expansion possibility, but no present conditions; red: future retraction and occurrence conditions only in the present (TIF 111192 kb)
10841_2022_407_MOESM3_ESM.tif
Figure S3. Maps of the future potential direction change for the butterfly species and their respective host plants: a) Battus polystictus, b) Aristolochia macroura, c) Aristolochia arcuata, d) Aristolochia galeata, e) Aristolochia gigantea, f) Aristolochia melastoma, g) Aristolochia triangularis predicted by the 5% threshold and derived from three coupled Atmosphere-Ocean General Circulation Models (AOGCM). Arrow color indication: black – RCP4.5 optimistic scenario; red - RCP8.5 pessimistic scenario (TIF 91213 kb)
10841_2022_407_MOESM4_ESM.tif
Figure S4. Overlap between the potential distribution of Battus polystictus and its host plants at present and under two future CO2 emissions scenario (RCP4.5, optimistic and RCP8.5, pessimistic) derived from three coupled Atmosphere-Ocean General Circulation Models (AOGCM). Host plants: a) Aristolochia gigantea; b) Aristolochia galeata; c) Aristolochia arcuata. Colors indicate estimated potential distribution of species and potential interactions: Blue: B. polystictus; Red: host plant; Green: overlap between butterfly and host plant potential distribution (TIF 96200 kb)
Rights and permissions
About this article
Cite this article
Bellaver, J.M.F., Lima-Ribeiro, M.d., Hoffmann, D. et al. Rare and common species are doomed by climate change? A case study with neotropical butterflies and their host plants. J Insect Conserv 26, 651–661 (2022). https://doi.org/10.1007/s10841-022-00407-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-022-00407-1