Abstract
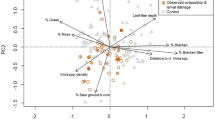
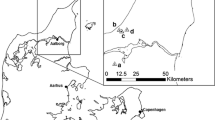
Knowledge of species’ ecological requirements is key for designing effective conservation management. In butterflies, the needs of larval stages are often the most specialised part of the life-cycle, but for many species information on this is lacking. The Mountain Ringlet Erebia epiphron is a cold-adapted butterfly found in alpine grasslands in mountainous regions of Europe. Efforts to devise conservation strategies for this climate change-threatened species are hampered due to its basic ecology being poorly understood. Here, we describe a study on the autecology of Mountain Ringlets at sites across its British distribution, focusing on the habitat preferences of egg-laying females as a proxy for larval preferences. Female Mountain Ringlets placed their eggs predominantly on Nardus stricta and Festuca ovina, but also on several other host plant species, suggesting larvae may be more broadly polyphagous than previously realised. Sites chosen for eggs had higher abundance of larval host plants, intermediate leaf litter cover, and lower cover of grass tussocks than random locations, as well as a shorter and sparser grass sward. Although the main host plant is ubiquitous in upland areas of Britain, our findings suggest that this butterfly’s egg and larval stages have specialised ecological requirements, requiring specific microhabitat features characterised by a narrow range of vegetation composition and structural characteristics. Many habitat associations are liable to be explicable as adaptations to ensure placement of eggs and larvae in sites within optimal (warm or buffered) microclimates. We tentatively suggest that the distribution of Mountain Ringlets in the landscape is thermally-constrained.



Similar content being viewed by others
References
Asher J, Warren M, Fox R, Harding P, Jeffcoate G, Jeffcoate S (2001) The millennium atlas of butterflies in Britain and Ireland. Oxford University Press, Oxford
Barton K (2018) MuMIn: multi-model inference. R package version 1.42.1
Bayfield N, McGowan GM, Taylor P, French DD, Mackie E, Waterhouse C, Hartley S (1995) Small Mountain Ringlet project: field studies rearing programme and questionaire survey 1994-95. Institute of Terrestrial Ecology
Boyd-Wallis W (1994) Survey of Creag Meagaidh NNR for colonies of Small Mountain Ringlet butterfly. Scottish Natural Heritage Report
Britton AJ, Beale CM, Towers W, Hewison RL (2009) Biodiversity gains and losses: evidence for homogenisation of Scottish alpine vegetation. Biol Conserv 142:1728–1739
Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Machler M, Bolker BM (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 9:378–400
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York
Čelik T, Vreš B (2018) Microtopography determines the habitat quality of a threatened peatland butterfly at its southern range margin. J Insect Conserv 22:707–720
Curtis RJ, Brereton TM, Dennis RL, Carbone C, Isaac NJ (2015) Butterfly abundance is determined by food availability and is mediated by species traits. J Appl Ecol 52:1676–1684
Davies ZG, Wilson RJ, Brereton TM, Thomas CD (2005) The re-expansion and improving status of the silver-spotted skipper butterfly (Hesperia comma) in Britain: a metapopulation success story. Biol Conserv 124:189–198
Dennis P, Skartveit J, McCracken DI, Pakeman RJ, Beaton K, Kunaver A, Evans DM (2008) The effects of livestock grazing on foliar arthropods associated with bird diet in upland grasslands of Scotland. J Appl Ecol 45:279–287
Dennis RL, Shreeve TG, Van Dyck H (2003) Towards a functional resource-based concept for habitat: a butterfly biology viewpoint. Oikos 102:417–426
Dormann CF, Elith J, Bacher S, Buchmann C, Carl G, Carre G, Marquez JRG, Gruber B, Lafourcade B, Leitao PJ, Munkemuller T, McClean C, Osborne PE, Reineking B, Schroder B, Skidmore AK, Zurell D, Lautenbach S (2013) Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36:27–46
Eichel S, Fartmann T (2008) Management of calcareous grasslands for Nickerl’s fritillary (Melitaea aurelia) has to consider habitat requirements of the immature stages, isolation, and patch area. J Insect Conserv 12:677–688
ESRI (2017) ArcGIS desktop. In: Release 10.5.1. Environmental Systems Research Institute, Redlands
Evans DM, Villar N, Littlewood NA, Pakeman RJ, Evans SA, Dennis P, Skartveit J, Redpath SM (2015) The cascading impacts of livestock grazing in upland ecosystems: a 10-year experiment. Ecosphere 6:1–15
Fartmann T, Hermann G (2006) Larvalökologie von Tagfaltern und Widderchen in Mitteleuropa–von den Anfängen bis heute. Abh Westf Mus Naturk 68:11–57
Fox R, Brereton T, Asher J, August T, Botham M, Bourn N, Cruickshanks K, Bulman C, Ellis S, Harrower C (2015) The state of the UK’s Butterflies 2015. Butterfly Conservation & Centre for Ecology and Hydrology, Wareham, Dorset
Franco AM, Hill JK, Kitschke C, Collingham YC, Roy DB, Fox R, Huntley B, Thomas CD (2006) Impacts of climate warming and habitat loss on extinctions at species' low-latitude range boundaries. Glob Change Biol 12:1545–1553
Frohawk FW (1924) Natural history of British butterflies. London
Galipaud M, Gillingham MA, Dechaume-Moncharmont FX (2017) A farewell to the sum of Akaike weights: the benefits of alternative metrics for variable importance estimations in model selection. Methods Ecol Evol 8:1668–1678
García-Barros E, Fartmann T (2009) Butterfly oviposition: sites, behaviour and modes. In: Settele J, Shreeve TG, Konvička M, Van Dyck H (eds) Ecology of butterflies in Europe. Cambridge University Press, Cambridge, pp 29–42
Grant SA, Torvell L, Sim EM, Small J, Armstrong R (1996) Controlled grazing studies on Nardus grassland: effects of between-tussock sward height and species of grazer on Nardus utilization and floristic composition in two fields in Scotland. J Appl Ecol 33:1053–1064
Hanski I (1998) Metapopulation dynamics. Nature 396:41–49
Hartig F (2018) DHARMa: residual diagnostics for hierarchical (Multi-Level/Mixed) regression models. R package version 0.2.0
Hill MO, Gauch HG (1980) Detrended correspondence analysis: an improved ordination technique. In: van der Maarel E (ed) Classification and ordination. Advances in vegetation science. Springer, Dordrecht, pp 47–58
Holm S (1979) A simple sequentially rejective multiple test procedure. Scand J Stat 6:65–70
Johnson CJ, Nielsen SE, Merrill EH, McDonald TL, Boyce MS (2006) Resource selection functions based on use–availability data: theoretical motivation and evaluation methods. J Wildl Manag 70(2):347–357
Konvička M, Beneš J, Kuras T (2002) Microdistribution and diurnal behaviour of two sympatric mountainous butterflies (Erebia epiphron and E. euryale): relations to vegetation and weather. Biol Bratisl 57:225–235
Konvicka M, Benes J, Cízek O, Kuras T, Klecková I (2016) Has the currently warming climate affected populations of the mountain ringlet butterfly, Erebia epiphron (Lepidoptera: Nymphalidae), in low-elevation mountains? Eur J Entomol 113:295
Kudrna O, Harpke A, Lux K, Pennerstorfer J, Schweiger O, Settele J, Wiemers M (2011) Distribution atlas of butterflies in Europe. Gesellschaft für Schmetterlingsschutz eV Halle
Kuras T, Beneš J, Konvička M, Honč L (2001) Life histories of Erebia sudetica sudetica and E. epiphron silesiana with description of immature stages (Lepidoptera Nymphalidae, Satyrinae). Atalanta 32:187–196
Kuras T, Benes J, Fric Z, Konvicka M (2003) Dispersal patterns of endemic alpine butterflies with contrasting population structures: Erebia epiphron and E. sudetica. Popul Ecol 45:115–123
Kuussaari M, van Nouhuys S, Hellmann JJ, Singer MC (2004) Larval biology of checkerspots. In: Ehrlich PR, Hanski I (eds) On the wings of checkerspots: a model system for population biology. Oxford University Press, Oxford
Massey FP, Hartley SE (2009) Physical defences wear you down: progressive and irreversible impacts of silica on insect herbivores. J Anim Ecol 78:281–291
Massey FP, Ennos AR, Hartley SE (2006) Silica in grasses as a defence against insect herbivores: contrasting effects on folivores and a phloem feeder. J Anim Ecol 75:595–603
Masterman (2008) The distribution and habitat of Mountain Ringlet Erebia epiphron (Knoch) in Scotland. Atropos 41:10–18
Mattila N, Kaitala V, Komonen A, Paivinen J, Kotiaho JS (2011) Ecological correlates of distribution change and range shift in butterflies. Insect Conserv Divers 4:239–246
Oksanen J, Blanchet FG, Kindt R, Legendre P, O’hara R, Simpson GL, Solymos P, Stevens MHH, Wagner H (2010) Vegan: community ecology package. R package version 1.17-4. http://cran.r-project.org
Pakeman R, Hulme P, Torvell L, Fisher J (2003) Rehabilitation of degraded dry heather [Calluna vulgaris (L.) Hull] moorland by controlled sheep grazing. Biol Conserv 114:389–400
Scalercio S, Bonacci T, Mazzei A, Pizzolotto R, Brandmayr P (2014) Better up, worse down: bidirectional consequences of three decades of climate change on a relict population of Erebia cassioides. J Insect Conserv 18:643–650
Schmitt T, Louy D, Zimmermann E, Habel JC (2016) Species radiation in the Alps: multiple range shifts caused diversification in Ringlet butterflies in the European high mountains. Org Divers Evol 16:791–808
Shannon S (1995) A study of the Small Mountain Ringlet butterfly Erebia epiphron Knoch. South West Cumbria. University of Leicester, Leicester
Slamova I, Klecka J, Konvicka M (2013) Woodland and grassland mosaic from a butterfly perspective: habitat use by Erebia aethiops (L. epidoptera: S. atyridae). Insect Conserv Divers 6:243–254
Stewart K, Bourn N, Thomas J (2001) An evaluation of three quick methods commonly used to assess sward height in ecology. J Appl Ecol 38:1148–1154
Stuhldreher G, Fartmann T (2014) When habitat management can be a bad thing: effects of habitat quality, isolation and climate on a declining grassland butterfly. J Insect Conserv 18:965–979
Stuhldreher G, Fartmann T (2015) Oviposition-site preferences of a declining butterfly Erebia medusa (Lepidoptera: Satyrinae) in nutrient-poor grasslands. Eur J Entomol 112:493–499
Ter Braak CJ (1986) Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis. Ecology 67:1167–1179
Thomas J, Thomas C, Simcox D, Clarke R (1986) Ecology and declining status of the silver-spotted skipper butterfly (Hesperia comma) in Britain. J Appl Ecol 23:365–380
Thomas JA, Simcox D, Clarke RT (2009) Successful conservation of a threatened Maculinea butterfly. Science 325:80–83
Thomas CD, Hill JK, Anderson BJ, Bailey S, Beale CM, Bradbury RB, Bulman CR, Crick HQ, Eigenbrod F, Griffiths HM (2011a) A framework for assessing threats and benefits to species responding to climate change. Methods Ecol Evol 2:125–142
Thomas J, Simcox D, Hovestadt T (2011b) Evidence based conservation of butterflies. J Insect Conserv 15:241–258
Trivedi MR, Morecroft MD, Berry PM, Dawson TP (2008) Potential effects of climate change on plant communities in three montane nature reserves in Scotland, UK. Biol Conserv 141:1665–1675
Van Noordwijk C, Flierman DE, Remke E, WallisDeVries MF, Berg MP (2012) Impact of grazing management on hibernating caterpillars of the butterfly Melitaea cinxia in calcareous grasslands. J Insect Conserv 16:909–920
van Strien AJ, van Swaay CA, van Strien-van WT, Liempt MJ, Poot, WallisDeVries MF (2019) Over a century of data reveal more than 80% decline in butterflies in the Netherlands. Biol Conserv 234:116–122
Van Swaay C, Maes D, Warren M (2009) Conservation status of European butterflies. In: Settele J, Shreeve TG, Konvička M, van Dyck H (eds) Ecology of butterflies in Europe. Cambridge University Press, Cambridge, pp 322–338
WallisDeVries MF (2006) Larval habitat quality and its significance for the conservation of Melitaea cinxia in northwestern Europe. In: Fartmann T, Hermann G (eds) Larvalökologie von Tagfaltern und Widderchen in Mitteleuropa. Abhandlungen aus dem Westfälischen Museum für Naturkunde, Münster, Germany, pp 281–294
Warren M (1991) The successful conservation of an endangered species, the heath fritillary butterfly Mellicta athalia, in Britain. Biol Conserv 55:37–56
Warren M, Hill J, Thomas J, Asher J, Fox R, Huntley B, Roy D, Telfer M, Jeffcoate S, Harding P (2001) Rapid responses of British butterflies to opposing forces of climate and habitat change. Nature 414:65–69
Weking S, Hermann G, Fartmann T (2013) Effects of mire type, land use and climate on a strongly declining wetland butterfly. J Insect Conserv 17:1081–1091
Wheeler AS (1982) Erebia epiphron Knoch (Lep., Satyridae) reared on a two-year life cycle. Proc Br Entomol Nat Hist Soc 15:28
Wiklund C (1984) Egg-laying patterns in butterflies in relation to their phenology and the visual apparency and abundance of their host plants. Oecologia 63:23–29
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1:3–14
Acknowledgements
We would like to thank James Gordon, Mark Yeldham, Ewan Munro, Mark Whiffin and Pete Howard for their assistance with data collection in the field. Thanks are also due to Martin Tordoff from the Butterfly Conservation Cumbria Branch, Dave Wainwright from Butterfly Conservation, John Hooson and Matthew Oates from the National Trust, and Helen Cole from the National Trust for Scotland for help designing this project, arranging access permissions and generally providing support and assistance. Funding for this work was provided by the Royal Society for the Protection of Birds. We also thank the generosity of 55 supporters who contributed donations to our ‘Giving Mountain Ringlets a brighter future’ crowdfunding campaign. Earlier drafts of this paper were substantially improved by comments from Graeme Buchanan, Jerry Wilson and two anonymous referees.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that there are no conflicting or competing interests.
Research involving human participants
This research did not involve human participants requiring ethics approval.
Rights and permissions
About this article
Cite this article
Ewing, S.R., Menéndez, R., Schofield, L. et al. Vegetation composition and structure are important predictors of oviposition site selection in an alpine butterfly, the Mountain Ringlet Erebia epiphron. J Insect Conserv 24, 445–457 (2020). https://doi.org/10.1007/s10841-020-00229-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-020-00229-z