Abstract
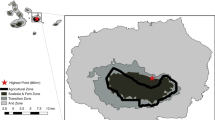
Understanding the factors that determine habitat quality is of vital importance in ensuring appropriate habitat management. Here we used the Niobe fritillary (Argynnis niobe) as a study system to analyse the larval habitat preferences in a small network of heavy-metal grasslands in western Germany. The data were compared with the results of a previous study in coastal dune grasslands of the German North Sea. Based on this knowledge, we give management recommendations for the conservation of this threatened species. The key factors for the survival of A. niobe in heavy-metal grasslands were (i) open vegetation with a warm microclimate and (ii) sufficient host plants for the larvae. This reflects similar results from the previous study in coastal grey dune grasslands. However, in the heavy-metal grasslands, physiological stress generally slows down succession and favours the fritillary’s host plant, the metallophyte Viola calaminaria. As a result, the cover of the host plant was nearly twice as high in heavy-metal grasslands compared to the dune grasslands. Heavy-metal grasslands are of great significance for the conservation of A. niobe and overall butterfly diversity. Usually, the speed of succession in heavy-metal grasslands is slow and, hence, sites with high heavy-metal concentrations are characterised by relatively stable plant composition and vegetation structure. However, on soils with low heavy-metal content a loss of habitats of A. niobe and associated species of conservation concern may occur without management. On those sites sheep grazing seems to be an appropriate way to keep the habitats open and rich in violets.

Data sources: Bräu et al. (2013), Brockmann (1989), Ebert and Rennwald (1991), Föhst and Broszkus (1992), Kraus (1993), Lederer and Künnert (1963), Lobenstein (2003), NLWKN (2006), Reinhardt (1983, 2005), Stamm (1981) as well as Aquazoo – Löbbecke Museum, H. Andretzke, S. Buchholz, S. Caspari, J. Gelbrecht, S. Hafner, H. G. Joger, J. Kleinekuhle, D. Koelman, D. Kolligs, A. C. Lange, D. Lück, P. Mansfeld, B. Nannen, A. Nunner, R. Ohle, R. Reinhardt, F. Röbbelen, A. Schmidt, P. Schmidt, M. Sommerfeld, R. Trusch (all pers. comm.)


Similar content being viewed by others
References
Anthes N, Fartmann T, Hermann G, Kaule G (2003) Combining larval habitat quality and metapopulation structure—the key for successful management of pre-alpine Euphydryas aurinia colonies. J Insect Conserv 7:175–185
Beneš J, Kepka P, Konvička M (2002) Limestone quarries as refuges for European xerophilous butterflies. Conserv Biol 17:1058–1069
Bink FA (1992) Ecologische atlas van de dagvlinders van Noordwest-Europa. Schuyt, Haarlem
Bos FG, Bosveld MA, Groenendijk DG, van Swaay CAM, Wynhoff I (2006) De dagvlinders van Nederland. Verspreiding en bescherming. Nederlandse Fauna 7. KNNV Uitgeverij, Leiden
Bräu M, Bolz R, Kolbeck H, Nunner A, Voith J, Wolf W (2013) Tagfalter in Bayern. Eugen Ulmer, Stuttgart
Brockmann, E (1989) Schutzprogramm für Tagfalter in Hessen (Papilionoidea und Hesperioidea). Stiftung Hessischer Naturschutz, Reiskirchen
Brown G (1993) Pflanzensoziologische, vegetationsökologische und ökophysiologische Untersuchungen der Schwermetallrasen der Eifel. Dissertation, Rheinische-Friedrich-Wilhelms-Universität Bonn
Chapin FS, Zavaleta ES, Eviner VT, Naylor RL, Vitousek PM, Reynolds HL, Hooper DU, Lavorel S, Sala OE, Hobbie SE (2000) Consequences of changing biodiversity. Nature 405:234–242
De Vos JM, Joppa LN, Gittleman JL, Stephens PR, Pimm SL (2014) Estimating the normal background rate of species extinction. Conserv Biol 29:452–462
Dennis RLH, Shreeve TG, van Dyck H (2006) Habitats and resources: the need for a resource-based definition to conserve butterflies. Biodivers Conserv 15:1943–1966
DWD (Deutscher Wetterdienst) (2016a) Langjährige Mittelwerte. http://www.dwd.de. Accessed 24 Sep 2016
DWD (Deutscher Wetterdienst) (2016b) Archiv Monats- und Tageswerte. http://www.dwd.de. Accessed 24 Sep 2016
Ebert G, Rennwald E (1991) Die Schmetterlinge Baden-Württembergs. Band 1, Tagfalter I. Eugen Ulmer, Stuttgart
EC (European Commission) (2007) Interpretation manual of European union habitats—EUR27. European Commission, DG Environment, Brussels
Eichel S, Fartmann T (2008) Management of calcareous grasslands for Nickerl’s fritillary (Melitaea aurelia) has to consider habitat requirements of the immature stages, isolation and patch area. J Insect Conserv 12:677–688
Ernst W (1974) Schwermetallvegetation der Erde. Gustav Fischer Verlag, Stuttgart
Fartmann T (2004) Die Schmetterlingsgemeinschaften der Halbtrockenrasen-Komplexe des Diemeltales. Abh Westf Mus Naturkde 66:1–256
Fartmann T (2006) Welche Rolle spielen Störungen für Tagfalter und Widderchen?. In: Fartmann T, Hermann G (eds) Larvalökologie von Tagfaltern und Widderchen in Mitteleuropa, vol 68. Abh Westf Mus Naturkde, Münster, pp 259–270
Fartmann T, Hermann G (2006) Larvalökologie von Tagfaltern und Widderchen in Mitteleuropa—von den Anfängen bis heute. In: Fartmann T, Hermann G (eds) Larvalökologie von Tagfaltern und Widderchen in Mitteleuropa, vol 68. Abh Westf Mus Naturkde, Münster, pp 11–57
Föhst P, Broszkus W (1992) Beiträge zur Kenntnis der Schmetterlingsfauna (Insecta: Lepidoptera) des Hunsrück-Nahe-Gebiets (BRD), Rheinland-Pfalz. Fauna und Flora in Rheinland-Pfalz Beih, vol 3. Ges. für Naturschutz und Ornithologie Rheinland-Pfalz, Mainz, pp 5–334
Fric Z, Konvička M (2002) Perleťovec maceškový Argynnis niobe (Linnaeus, 1758). In: Beneš J, Konvička M, Dvořak J, Fric Z, Havelda Z, Pavlíčko A, Vrabec V, Weidenhoffer Z (eds) Motýli České republiky: Rozšiření a ochrana I. Butterflies of the Czech Republic: distribution and Conservation I. SOM, Prague, pp 409–411
García-Barros E, Fartmann T (2009) Butterfly oviposition: sites, behaviour and modes. In: Settele J, Konvicka M, Shreeve T, van Dyck H (eds) Ecology of butterflies in Europe. Cambridge University Press, Cambridge, pp 29–42
Hafner S (2005) Neue Beobachtungen zum Vorkommen von Fabriciana niobe im Schwarzwald und auf der Schwäbischen Alb. In: Ebert G (ed) Die Schmetterlinge Baden-Württembergs. Band 10, Ergänzungsband. Eugen Ulmer, Stuttgart, pp 46–47
Krämer B, Kämpf I, Enderle J, Poniatowski D, Fartmann T (2012) Microhabitat selection in a grassland butterfly: a trade-off between microclimate and food availability. J Insect Conserv 16:857–865
Kraus W (1993) Verzeichnis der Großschmetterlinge (Insecta: Lepidoptera) der Pfalz. Selbstverlag, Bad Dürkheim
LANUV (Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen) (2016) Excerpt from the habitat register of North Rhine-Westphalia. Accessed 24 Sep 2016
Lederer G, Künnert R (1963) Beiträge zur Insektenfauna des Mittelrheins und der angrenzenden Gebiete. Entomol Z 73:237–243
Leopold P (2006) Larvalökologie der Rostbinde Hipparchia semele (Linnaeus, 1758; Lepidoptera, Satyrinae) in Nordrhein-Westfalen. Die Notwendigkeit raumzeitlicher Störungsprozesse für den Arterhalt. Dissertation, Westfälische Wilhelms-Universität Münster
Lobenstein U (2003) Die Schmetterlingsfauna des mittleren Niedersachsens. Bestand, Ökologie und Schutz der Großschmetterlinge in der Region Hannover, der Südheide und im unteren Weser-Leine-Bergland. NABU, Bonn
Löffler F, Stuhldreher G, Fartmann T (2013) How much care does a shrub-feeding hairstreak butterfly, Satyrium spini (Lepidoptera: Lycaenidae), need in calcareous grasslands?. Eur J Entomol 110:145–152
Munguira M, García-Barros E, Cano JM (2009) Butterfly herbivory and larval ecology. In: Settele J, Shreeve TG, Konvicka M, van Dyck H (eds) Ecology of butterflies in Europe. Cambridge University Press, Cambridge, pp 43–54
NLWKN (Niedersächsischer Landesbetrieb für Wasserwirtschaft, Küsten- und Naturschutz) (ed) (2006) Argynnis niobe. Auszug aus den Funddaten des Tierarten-Erfassungsprogramms der Fachbehörde für Naturschutz. Stand: 13.03.2016
Noret N, Josens G, Escarre J, Lefebvre C, Panichelli S, Meerts P (2007) Development of Issoria lathonia (Lepidoptera: Nymphalidae) on zinc accumulating and nonaccumulating Viola species (Violaceae). Environ Tox Chem 26:565–571
Pardey A (1999) Grundlagen des Naturschutzes auf Schwermetallstandorten in Nordrhein-Westfalen. Abiotische Verhältnisse, Flora, Vegetation, Fauna aktuelle Schutzsituation und zukünftige Zielsetzungen. In: LANUV (ed) Naturschutzrahmenkonzeption Galmeifluren NRW. LÖBF-Schriftenr Bd 16:7–48
Pardey A, Hacker E, Schippers B (1999) Schutzgebiets- und Biotopverbundplanung für Schwermetallstandorte im Raum Aachen-Stolberg (Nordeifel). In: LANUV (ed) Naturschutzrahmenkonzeption Galmeifluren NRW. LÖBF-Schriftenr. Bd 16:99–128
Petersen J, Pott R (2005) Ostfriesische Inseln. Landschaft und Vegetation im Wandel. Schriften zur Heimatpflege. Veröffentl Niedersächs Heimatbd. 15:1–160
Raskin R (2008) Möglichkeiten und Grenzen der Regeneration von Schwermetallfluren. In: Lennartz G (ed) Renaturierung: Programmatik und Effektivitätsmessung. Academia Verlag, Sankt Augustin, pp 60–76
Reinhardt R (1983) Beiträge zur Insektenfauna der DDR. Lepidoptera—Rhopalocera et Hesperiidae. Entomol Nachr Ber, Beih 2
Reinhardt R (2005) Beiträge zur Tagfalterfauna Sachsens. Teil 2: Familie Nymphalidae (Edelfalter)—Unterfamilien Heliconiinae und Nymphalinae. Mitteilungen Sächs Entomol Suppl 3:1–210
Reinhardt R, Bolz R (2011) Rote Liste und Gesamtartenliste der Tagfalter (Rhopalocera) (Lepidoptera: Papilionoidea et Hesperioidea) Deutschlands. Natursch Biol Vielfalt 70:167–194
Sala OE, Chapin FS, Armesto JJ, Berlow E, Bloomfield J, Dirzo R, Huber-Sanwald E, Huenneke LF, Jackson RB, Kinzig A, Leemans R, Lodge DM, Mooney HA, Oesterheld M, Poff NL, Sykes MT, Walker BH, Walker M, Wall DH (2000) Global biodiversity scenarios for the year 2100. Science 287:1770–1774
Salz A, Fartmann T (2009) Coastal dunes as important strongholds for the survival of the rare Niobe fritillary (Argynnis niobe). J Insect Conserv 13:643–654
SBN (Schweizerischer Bund für Naturschutz – Lepidopteren-Arbeitsgruppe) (ed) (1987) Tagfalter und ihre Lebensräume. Arten, Gefährdung, Schutz. Fotorar AG, Egg/ZH
Spitzer L, Beneš J, Konvička M (2009) Oviposition of the Niobe fritillary (Argynnis niobe (Linnaeus, 1758)) at submountain conditions in the Czech Carpathians (Lepidoptera, Nymphalidae). Nachr Entomol Ver Apollo 30:165–168
Stamm K (1981) Prodomus der Lepidopteren-Fauna der Rheinlande und Westfalens. Selbstverlag, Solingen
Stoutjesdijk P, Barkman JJ (1992) Microclimate, vegetation and fauna. Opulus Press, Uppsala
Stuhldreher G, Fartmann T (2014) When habitat management can be a bad thing: effects of habitat quality, isolation and climate on a declining grassland butterfly. J Insect Conserv 18:965–979
Thomas JA (1991) Rare species conservation: case studies of European butterflies. In: Spellerberg IF, Goldsmith FB, Morris MG (eds) The scientific management of temperate communities for conservation. Blackwell Scientific, Oxford, pp 149–197
Thomas JA (2005) Monitoring change in the abundance and distribution of insects using butterflies and other indicator groups. Philos Trans Roy Soc B 360:339–357
Thomas JA, Clarke RT (2004) Extinction rates and butterflies. Science 305:1563–1564
Thomas JA, Simcox DJ, Wardlaw JC, Elmes GW, Hochberg ME, Clarke RT (1998) Effects of latitude, altitude and climate on the habitat and conservation of the endangered butterfly Maculinea arion and its Myrmica ant hosts. J Insect Conserv 2:39–46
Thomas JA, Bourn NAD, Clarke RT, Stewart KE, Simcox DJ, Pearman GS, Curtis R, Goodger B (2001) The quality and isolation of habitat patches both determine where butterflies persist in fragmented landscapes. Proc Roy Soc B 268:1791–1796
Thomas JA, Telfer MG, Roy DB, Preston CD, Greenwood JJD, Asher J, Fox R, Clarke RT, Lawton JH (2004) Comparative losses of British butterflies, birds and plants and the global extinction crisis. Science 303:1879–1881
Thomas JA, Simcox DJ, Hovestadt T (2011) Evidence based conservation of butterflies. J Insect Conserv 15:241–258
Tilman D, Fargione J, Wolff B, D’Antonio C, Dobson A, Howarth R, Schindler D, Schlesinger WH, Simberloff D, Swackhamer D (2001) Forecasting agriculturally driven global environmental change. Science 292:281–284
Tonne F (1954) Besser Bauen mit Besonnungs- und Tageslichtplanung. Hofmann, Schondorf
Walker BH (1992) Biodiversity and ecological redundancy. Conserv Biol 6:18–23
WallisDeVries MF (2004) A quantitative conservation approach for the endangered butterfly Maculinea alcon. Conserv Biol 18:488–499
WallisDeVries MF (2006) Larval habitat quality and its significance for the conservation of Melitaea cinxia in northwestern Europe. In: Fartmann T, Hermann G (eds) Larvalökologie von Tagfaltern und Widderchen in Mitteleuropa. Abh Westf Mus Naturkde 68:281–294
Warren MS (1993) A review of butterfly conservation in central southern Britain: II. Site management and habitat selection of key species. Biol Conserv 64:37–49
Acknowledgements
We are grateful to J. Dover, C. Haaland, D. Lück, B. Theißen and two anonymous reviewers for valuable comments on an earlier version of the manuscript. Moreover, we would like to thank D. Lück and A. Deepen-Wiezcorek for providing information about the butterfly assemblages in the heavy-metal grasslands around Stollberg. We are also grateful to R. Altmüller, H. Andretzke, R. Bolz, M. Bräu, S. Buchholz, S. Caspari, J. Gelbrecht, F. Goosmann, S. Hafner, H. G. Joger, J. Kleinekuhle, D. Koelman, D. Kolligs, A. C. Lange, D. Lück, P. Mansfeld, B. Nannen, R. Ohle, R. Reinhardt, F. Röbbelen, A. Schmidt, P. Schmidt, M. Sommerfeld, R. Trusch, J. Voith and H. Wegner for providing distribution data of Argynnis niobe.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salz, A., Fartmann, T. Larval habitat preferences of a threatened butterfly species in heavy-metal grasslands. J Insect Conserv 21, 129–136 (2017). https://doi.org/10.1007/s10841-017-9961-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-017-9961-7