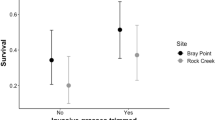
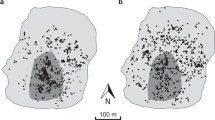
Abstract
Accurate estimates of population size are essential for effective conservation and restoration management of threatened species. Nevertheless, reliable methods to estimate population size, such as mark-release-recapture studies (MRR), are time and labour consuming and may generate negative impact(s) on both the habitats and organisms studied. This may complicate their use if several sites need to be studied concurrently. Consequently, there is a strong interest to develop reliable proxies of population size, e.g., to be used in Population Viability Analysis. Habitat area has often been used as an obvious proxy. For butterflies, many studies focused on the area of host plant patches, but resource-based definition of the habitat (i.e., the area containing the different ecological resources and conditions needed by the individuals) has recently gained much attention. Using two peat bog butterflies, we tested the reliability of these two measures of habitat area as proxies for population size by (1) predicting population sizes based on the product of larval habitat area by the number of emerged butterflies per spatial unit of habitat (eliminated by ground cover traps) and (2) comparing these predictions to accurate population size estimates inferred from MRR studies. Results on both species showed that: (1) adult population size was strongly related to larval habitat availability and quality when habitat was accurately defined according to functional resources, (2) resources other than the host plant have to be included in the habitat definition, (3) after careful control of its similarity, the resource-based habitat delineation can be reasonably well transferred among populations of the same species in a wider region.



Similar content being viewed by others
References
Baguette M (2003) Long distance dispersal and landscape occupancy in a metapopulation of the cranberry fritillary butterfly. Ecography 26:153–160
Baguette M, Nève G (1994) Adult movements between populations in the specialist butterfly Proclossiana eunomia (Lepidoptera, Nymphalidae). Ecol Entomol 19:1–5
Begon M, Townsend CR, Harper JL (2006) Ecology, from individuals to ecosystems. Blackwell Publishing, Malden, MA
Belovsky GE, Mellison C, Larson C, Van Zandt PA (1999) Experimental studies of extinction dynamics. Science 286:1175–1177
Bink FA (1992) Ecologische atlas van de dagvlinders van Noordwest-Europa. Schuyt & Co, Haarlem
Binzenhofer B, Schroder B, Strauss B, Biedermann R, Settele J (2005) Habitat models and habitat connectivity analysis for butterflies and burnet moths—the example of Zygaena carniolica and Coenonympha arcania. Biol Conserv 126:247–259
Boyce MS, MacDonald LL (1999) Relating populations to habitats using resource selection functions. Trends Ecol Evol 14:268–272
Callaham MA Jr, Whiles MR, Meyer CK, Brock BL, Charlton RE (2000) Feeding ecology and emergence production of annual cicadas (Homoptera: Cicadidae) in tallgrass prairie. Oecologia 123:535–542
Cançado PHD, Piranda EM, Mourão GM, Faccini JLH (2008) Spatial distribution and impact of cattle-raising on ticks in the Pantanal region of Brazil by using the CO2 tick trap. Parasitol Res 103:371–377
Collier N, MacCay DA, Bekendorff K, Austin AD, Carthew SM (2006) Butterfly communities in South Australian urban reserves: estimating abundance and diversity using Pollard walk. Austral Ecology 31:282–290
Collier N, MacCay DA, Bekendorff K (2008) Is relative abundance a good estimator of population size? Evidence from fragmented populations of a specialist butterfly (Lepidoptera: Lycaenidae). Pop Ecol 50:17–23
Coulson T, Mace GM, Hudson E, Possingham H (2001) The use and abuse of population viability analysis. Trends Ecol Evol 16:219–221
Dennis RLH (2004) Just how important are structural elements as habitat components? Indications from a declining lycaenid butterfly with priority conservation status. J Insect Conserv 8:37–45
Dennis RLH, Shreeve TG, Van Dyck H (2003) Towards a functional resource-based concept for habitat : a butterfly biology viewpoint. Oikos 102:417–426
Dennis RLH, Shreeve TG, Van Dyck H (2006a) Habitats and resources: the need for a resource-based definition to conserve butterflies. Biodivers Conserv 15:1943–1966
Dennis RLH, Shreeve TG, Isaac NJB, Roy DB, Hardy PB, Fox R, Asher J (2006b) The effects of visual apparency on bias in butterfly recording and monitoring. Biol Conserv 128:486–492
Fahrig L (2001) How much habitat is enough. Biol Conserv 100:65–74
Fichefet V, Barbier Y, Baugnée J-Y, Dufrêne M, Goffart P, Maes D, Van Dyck H (2008) Papillons de jour de Wallonie (1985–2007). Publication du Groupe de Travail Papillons de jour Lycaena et du Centre de Recherche de la Nature, des Forêts et du Bois (MRW-DGRNE), Gembloux
Fischer K, Beinlich B, Plachter H (1999) Population structure, mobility and habitat preferences of the Violet Copper Lycaena helle (Lepidoptera: Lycaenidae) in Western Germany: implications for conservation. J Insect Conserv 3:43–52
Fischer DO, Blomberg SP, Owens IPF (2003) Extrinsic cersus intrinsic factors in the decline and extinction of Australian marsupials. Proc R Soc Lond (B) 270:1801–1808
Gall LF (1984) The effect of capturing and marking on subsequent activity in Boloria acrocnema (Lepidoptera: Nymphalidae), with a comparison of different numerical models that estimate population size. Biol Conserv 28:139–154
Garshelis DV (2000) Delusions in habitat evaluation: measuring use, selection and importance. In: Boitani L, Fuller TK (eds) Research techniques in animal ecology. Controversies and consequences. Columbia University Press, New York, pp 111–164
Goffart P, Baguette M, Dufrêne M, Mousson L, Nève G, Sawchik J, Weiserbs A, Lebrun P (2001) Gestion des milieux semi-naturels et restauration de populations menacées de papillons de jour
Goffart P, Schtickzelle N, Turlure C (2010) Conservation and management of the habitats of two relict butterflies in the Belgian Ardenne: Proclossiana eunomia & Lycaena helle. Relict species: phylogeography and conservation biology. Springer-Verlag, Berlin
Gross K, Kalendra EJ, Hudgens BR, Haddad NM (2007) Robustness and uncertainty in estimates of butterfly abundance from transects counts. Pop Ecol 49:191–200
Haddad NM, Hudgens BR, Damiani C, Gross K, Kuefler D (2007) Determining optimal population monitoring for rare butterflies. Conserv Biol 22:929–940
Harker R, Shreeve T (2008) How accurate are single site transect data for monitoring butterfly trends? Spatial and temporal issues identified in monitoring Lasiommata megera. J Insect Conserv 12:125–133
Jones KE, Purvis A, Gittleman JL (2003) Biological correlates of extinction risk in bats. Am Nat 161:601–614
Joseph LN, Field SA, Wilcox C, Possingham HP (2006) Presence-absence versus abundance data for monitoring threatened species. Conserv Biol 20:1679–1687
Kery M, Schmid H (2004) Monitoring programs need to take into account imperfect species detectability. Basic Appl Ecol 5:65–73
Kitahara M (2004) Butterfly community composition and conservation in and around a primary woodland of Mount Fuji, central Japan. Biodivers Conserv 13:917–932
Lewington R, Levins R (1989) On the characterisation of density and resource availability. Am Nat 134:513–524
Murphy DD (1988) Are we studying our endangered butterflies to death? Journal of Research on the Lepidoptera 26:236–239
Nowicki P, Settele J, Henry P-Y, Woyciechowski M (2008) Butterfly monitoring methods: the ideal and the real world. Israel J Ecol Evol 54:69–88
O’Grady JJ, Reed DH, Brook BW, Frankham R (2004) What are the best correlates of predicted extinction risk? Biol Conserv 118:513–520
Pollard E (1977) A method for assessing changes in the abundance of butterflies. Biol Conserv 12:115–134
Pollard E (1988) Temperature, rainfall and butterfly numbers. J Appl Ecol 25:819–828
Pollard E, Yates TJ (1993) Monitoring butterflies for ecology and conservation. Chapman & Hall, London
Randin CF, Dirnböck T, Dullinger S, Zimmerman NE, Zappa M, Guisan A (2006) Are niche-based species distribution models transferable in space? J Biogeogr 33:1689–1703
Sawchik J, Dufrêne M, Lebrun P, Schtickzelle N, Baguette M (2002) Metapopulation dynamics of the bog fritillary butterfly: modelling the effect of habitat fragmentation. Acta Oecologica 23:287–296
Schtickzelle N, Baguette M (2004) Metapopulation viability analysis of the bog fritillary butterfly using RAMAS/GIS. Oikos 104:277–290
Schtickzelle N, Baguette M (2009) (Meta) population viability analysis: a crystal ball for the conservation of endangered butterflies? In: Settele J, Shreeve TG, Konvicka M, Van Dyck H (eds) Ecology of butterflies in Europe. Cambridge University Press, Cambridge
Schtickzelle N, Le Boulengé E, Baguette M (2002) Metapopulation dynamics of the bog fritillary butterfly: demographic processes in a patchy population. Oikos 97:349–360
Schtickzelle N, WallisDeVries MF, Baguette M (2005) Using surrogate data in population viability analysis: the case of the critically endangered cranberry fritillary butterfly. Oikos 109:89–100
Schtickzelle N, Turlure C, Baguette M (2007) Grazing management impacts on the viability of the threatened bog fritillary butterfly: Proclossiana eunomia. Biol Conserv 136:651–660
Schultz CB (1998) Dispersal behavior and its implications for reserve design in a rare oregon butterfly. Conserv Biol 12:284–292
Schultz CB, Hammond PC (2003) Using population viability analysis to develop recovery criteria for endangered insects: Case study of the fender’s blue butterfly. Conserv Biol 17:1372–1385
Service MW (1993) Mosquito ecology. Field sampling methods. Chapman & Hall, London
Shaw MR, Stefanescu C, van Nouhuys S (2009) Parasitism of European butterflies (Hesperioidea and Papilionoidea). In: Settele J, Shreeve TG, Konvicka M, Van Dyck H (eds) Ecology of butterflies in Europe. Cambridge University Press, Cambridge
Singer MC (1972) Complex components of habitat suitability within a butterfly colony. Science 176:75–77
Singer MC, Wedlake P (1981) Capture does affect probability of recapture in a butterfly species. Ecol Entomol 6:215–216
Sjögren-Gulve P, Hanski I (2000) Metapopulation viability analysis using occupancy models. Ecological Bulletins 48:53–71
Soberon MJ (1986) The relationship between use and suitability of resources and its consequences to insect population size. Am Nat 127:338–357
Sutherland WJ (1996) Ecological census techniques, a handbook. Cambridge University Press, Cambridge
Thomas JA (1983) A quick method of assessing butterfly numbers during survey. Biol Conserv 27:195–211
Thomas JA (2005) Monitoring change in the abundance and distribution of insects using butterflies and other indicator groups. Philosophical Transactions of the Royal Society B: Biological Sciences 360:339–357
Turlure C, Van Dyck H, Schtickzelle N, Baguette M (2009) Resource-based definition of the habitat, niche overlap and conservation of two glacial relict butterflies. Oikos 118:950–960
Turlure C, Choutt J, Baguette M, Van Dyck H (2010) Microclimate buffering and resource-based habitat of a relict butterfly species: significance for conservation under climate change. Global Change Biology (in press)
Van Dyke F (2008) The conservation of populations: concept, theory and analysis. In: Van Dyke F (ed) Conservation biology. Foundations, concepts, applications. Springer, New York, pp 213–242
van Swaay C, Warren M (2006) Prime butterfly areas of Europe: An initial selection of priority sites for conservation. J Insect Conserv 10:5–11
Vanreusel W, Van Dyck H (2007) When functional habitat does not match vegetation types: a resource-based approach to map butterfly habitat. Biol Conserv 135:202–211
Vanreusel W, Maes D, Van Dyck H (2007) Transferability of species distribution models: a functional habitat approach for two regionally threatened butterflies. Conserv Biol 21:201–212
White GC (2000) Population viability analysis: data requirements and essential analysis. In: Boitani L, Fuller TK (eds) Research techniques in animal ecology. Controversies and consequences. Columbia University Press, New York, pp 288–331
White GC, Burnham KP (1999) Program MARK: survival estimation from populations of marked animals. Bird Study 46(Suppl):S120–S139
Williams BK, Nichols JD, Conroy MJ (2002) Analysis and management of animal populations: modelling, estimation and decision making. Academic press, San Diego, California
Zonneveld C, Longcore T, Mulder C (2003) Optimal schemes to detect the presence of insect species. Conserv Biol 17:476–487
Acknowledgments
We thank Philippe Goffart and Michel Pirnay for their valuable help with field work. C. T. was supported by a Ph.D. grant of the FRIA-fund. J. C. is teaching assistant at the UCL. N. S. is Research Associate of the F.R.S.-FNRS. Site access and a permission to study the species in the field and in the laboratory were granted by the Ministère de la Région Wallonne. This is publication BRC162 of the Biodiversity Research Centre at UCL.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Turlure, C., Choutt, J., Van Dyck, H. et al. Functional habitat area as a reliable proxy for population size: case study using two butterfly species of conservation concern. J Insect Conserv 14, 379–388 (2010). https://doi.org/10.1007/s10841-010-9269-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-010-9269-3