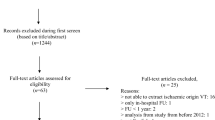
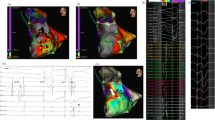
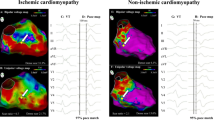
Abstract
Background
Recent studies showed that an early strategy for ventricular tachycardia (VT) ablation resulted in reduction of VT episodes or mortality. Cardiac magnetic resonance (CMR)-derived border zone channel (BZC) mass has proved to be a strong non-invasive predictor of VT in post-myocardial infarction (MI). CMR-guided VT substrate ablation proved to be safe and effective for reducing sudden cardiac death (SCD) and VA occurrence.
Methods
PREVENT-VT is a prospective, randomized, multicenter, and controlled trial designed to evaluate the safety and efficacy of prophylactic CMR-guided VT substrate ablation in chronic post-MI patients with CMR-derived arrhythmogenic scar characteristics. Chronic post-MI patients with late gadolinium enhancement (LGE) CMR will be evaluated. CMR images will be post-processed and the BZC mass measured: patients with a BZC mass > 5.15 g will be eligible. Consecutive patients will be enrolled at 3 centers and randomized on a 1:1 basis to undergo a VT substrate ablation (ABLATE arm) or optimal medical treatment (OMT arm). Primary prevention ICD will be implanted following guideline recommendations, while non-ICD candidates will be implanted with an implantable cardiac monitor (ICM). The primary endpoint is a composite outcome of sudden cardiac death (SCD) or sustained monomorphic VT, either treated by an ICD or documented with ICM. Secondary endpoints are procedural safety and efficiency outcomes of CMR-guided ablation.
Discussion
In some patients, the first VA episode causes SCD or severe neurological damage. The aim of the PREVENT-VT is to evaluate whether primary preventive substrate ablation may be a safe and effective prophylactic therapy for reducing SCD and VA occurrence in patients with previous MI and high-risk scar characteristics based on CMR.
Trial registration
ClinicalTrials.gov, NCT04675073, registered on January 1, 2021.


Similar content being viewed by others
Data availability
Not applicable. Enrollment is currently ongoing, and no datasets were generated for analysis yet.
Abbreviations
- BZC:
-
Border zone channel
- CL:
-
Cycle length
- EGM:
-
Electrogram
- EPS:
-
Electrophysiological study
- FAM:
-
Fast anatomical map
- ICM:
-
Implantable Cardiac Monitoring
- IHD:
-
Ischemic heart disease
- ICD:
-
Implantable cardioverter defibrillator
- LGE-CMR:
-
Late gadolinium enhancement-cardiac magnetic resonance
- LVEF:
-
Left ventricular ejection fraction
- MDCT:
-
Multi-detector computed tomography
- MI:
-
Myocardial infarction
- PSI:
-
Pixel signal intensity
- PVS:
-
Programmed ventricular stimulation
- SMVT:
-
Sustained monomorphic ventricular tachycardia
- TOE:
-
Transoesophageal echocardiogram
- TTE:
-
Transthoracic echocardiogram
- VA:
-
Ventricular arrhythmia
- VT:
-
Ventricular tachycardia
References
de Bakker JM, van Capelle FJ, Janse MJ, et al. Reentry as a cause of ventricular tachycardia in patients with chronic ischemic heart disease: electrophysiologic and anatomic correlation. Circulation. 1988;77:589–606.
Klem I, Weinsaft JW, Bahnson TD, et al. Assessment of myocardial scarring improves risk stratification in patients evaluated for cardiac defibrillator implantation. J Am Coll Cardiol. 2012;60:408–20.
Perez-David E, Arenal A, Rubio-Guivernau JL, et al. Noninvasive identification of ventricular tachycardia-related conducting channels using contrast-enhanced magnetic resonance imaging in patients with chronic myocardial infarction: comparison of signal intensity scar mapping and endocardial voltage mapping. J Am Coll Cardiol. 2011;57:184–94.
Fernandez-Armenta J, Berruezo A, Andreu D, et al. Three-dimensional architecture of scar and conducting channels based on high resolution ce-CMR: insights for ventricular tachycardia ablation. Circ Arrhythm Electrophysiol. 2013;6:528–37.
Jauregui B, Soto-Iglesias D, Penela D, et al. Cardiovascular magnetic resonance determinants of ventricular arrhythmic events after myocardial infarction. Europace. 2022;24(6):938–947.
Della Bella P, Baratto F, Vergara P, et al. Does timing of ventricular tachycardia ablation affect prognosis in patients with an implantable cardioverter defibrillator? Results From the Multicenter Randomized PARTITA Trial. Circulation. 2022;145(25):1829–1838.
Arenal A, Avila P, Jimenez-Candil J, et al. Substrate ablation vs antiarrhythmic drug therapy for symptomatic ventricular tachycardia. J Am Coll Cardiol. 2022;79:1441–53.
Poole JE, Johnson GW, Hellkamp AS, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359:1009–17.
Pannone L, Falasconi G, Cianfanelli L, et al. Sudden cardiac death in patients with heart disease and preserved systolic function: current options for risk stratification. J Clin Med. 2021;10(9):1823.
Gatzoulis KA, Vouliotis AI, Tsiachris D, et al. Primary prevention of sudden cardiac death in a nonischemic dilated cardiomyopathy population: reappraisal of the role of programmed ventricular stimulation. Circ Arrhythm Electrophysiol. 2013;6:504–12.
LaPointe NM, Al-Khatib SM, Piccini JP, et al. Extent of and reasons for nonuse of implantable cardioverter defibrillator devices in clinical practice among eligible patients with left ventricular systolic dysfunction. Circ Cardiovasc Qual Outcomes. 2011;4:146–51.
Fernandez-Armenta J, Soto-Iglesias D, Silva E, et al. Safety and outcomes of ventricular tachycardia substrate ablation during sinus rhythm: a prospective multicenter registry. JACC Clin Electrophysiol. 2020;6:1435–48.
Soto-Iglesias D, Penela D, Jauregui B, et al. Cardiac magnetic resonance-guided ventricular tachycardia substrate ablation. JACC Clin Electrophysiol. 2020;6:436–47.
Fernandez-Armenta J, Penela D, Acosta J, et al. Substrate modification or ventricular tachycardia induction, mapping, and ablation as the first step? A randomized study Heart Rhythm. 2016;13:1589–95.
Priori SG, Blomstrom-Lundqvist C, Mazzanti A, et al. ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36:2793–867.
Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–77.
Roca-Luque I, Van Breukelen A, Alarcon F, et al. Ventricular scar channel entrances identified by new wideband cardiac magnetic resonance sequence to guide ventricular tachycardia ablation in patients with cardiac defibrillators. Europace. 2020;22:598–606.
Andreu D, Ortiz-Perez JT, Fernandez-Armenta J, et al. 3D delayed-enhanced magnetic resonance sequences improve conducting channel delineation prior to ventricular tachycardia ablation. Europace. 2015;17:938–45.
Soto-Iglesias D, Acosta J, Penela D, et al. Image-based criteria to identify the presence of epicardial arrhythmogenic substrate in patients with transmural myocardial infarction. Heart Rhythm. 2018;15:814–21.
Acosta J, Andreu D, Penela D, et al. Elucidation of hidden slow conduction by double ventricular extrastimuli: a method for further arrhythmic substrate identification in ventricular tachycardia ablation procedures. Europace. 2018;20:337–46.
Acosta J, Fernandez-Armenta J, Borras R, et al. Scar Characterization to predict life-threatening arrhythmic events and sudden cardiac death in patients with cardiac resynchronization therapy: the GAUDI-CRT study. JACC Cardiovasc Imaging. 2018;11:561–72.
Anderson KP. Sudden cardiac death unresponsive to implantable defibrillator therapy: an urgent target for clinicians, industry and government. J Interv Card Electrophysiol. 2005;14:71–8.
Reddy VY, Reynolds MR, Neuzil P, et al. Prophylactic catheter ablation for the prevention of defibrillator therapy. N Engl J Med. 2007;357:2657–65.
Willems S, Tilz RR, Steven D, et al. Preventive or deferred ablation of ventricular tachycardia in patients with ischemic cardiomyopathy and implantable defibrillator (BERLIN VT): A Multicenter Randomized Trial. Circulation. 2020;141:1057–67.
Kuck KH, Schaumann A, Eckardt L, et al. Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): a multicentre randomised controlled trial. Lancet. 2010;375:31–40.
Kuck KH, Tilz RR, Deneke T, et al. Impact of substrate modification by catheter ablation on implantable cardioverter-defibrillator interventions in patients with unstable ventricular arrhythmias and coronary artery disease: results from the multicenter randomized controlled SMS (Substrate Modification Study). Circ Arrhythm Electrophysiol. 2017;10(3):e004422.
Schmidt A, Azevedo CF, Cheng A, et al. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation. 2007;115:2006–14.
Jauregui B, Soto-Iglesias D, Penela D, et al. Follow-up after myocardial infarction to explore the stability of arrhythmogenic substrate: the footprint study. JACC Clin Electrophysiol. 2020;6:207–18.
Acosta J, Fernandez-Armenta J, Penela D, et al. Infarct transmurality as a criterion for first-line endo-epicardial substrate-guided ventricular tachycardia ablation in ischemic cardiomyopathy. Heart Rhythm. 2016;13:85–95.
Funding
The study was partially funded by Biotronik, Germany.
Author information
Authors and Affiliations
Contributions
GF: study concept and design, manuscript drafting, and critical revision. DP: study concept and design, manuscript drafting, and critical revision. DSI: study concept and design, manuscript drafting, and critical revision. AB: study concept and design, manuscript drafting, and critical revision. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the local Ethics Committee.
Conflict of interest
A. Berruezo is a stockholder of Galgo Medical. D. Soto-Iglesias is an employee of Biosense Webster. A. Berruezo received speaker fees from Biosense and research grants from Biotronik. P. Francia received speaker fees, research, and educational grants from Boston Scientific and Abbott. The other authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Giulio Falasconi M.D. and Diego Penela M.D., Ph.D. equally contributed to the article and should be considered as shared first authors.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Falasconi, G., Penela, D., Soto-Iglesias, D. et al. Preventive substrate ablation in chronic post-myocardial infarction patients with high-risk scar characteristics for ventricular arrhythmias: rationale and design of PREVENT-VT study. J Interv Card Electrophysiol 66, 39–47 (2023). https://doi.org/10.1007/s10840-022-01392-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-022-01392-w