Abstract
Background
Stereotactic body radioablation therapy (SBRT) has recently been introduced with the ability to provide ablative energy noninvasively to arrhythmogenic substrate while reducing damage to normal cardiac tissue nearby and minimizing patients’ procedural risk. There is still debate regarding whether SBRT has a predominant effect in the early or late period after the procedure. We sought to assess the time course of SBRT’s efficacy as well as the value of using a blanking period following a SBRT session.
Methods
Eight patients (mean age 58 ± 14 years) underwent eight SBRT sessions for refractory ventricular tachycardia (VT). SBRT was given using a linear accelerator device with a total dose of 25 Gy to the targeted area.
Results
During a median follow-up of 8 months, all patients demonstrated VT recurrences; however, implantable cardioverter-defibrillator (ICD) and anti-tachycardia pacing therapies were significantly reduced with SBRT (8.46 to 0.83/per month, p = 0.047; 18.50 to 3.29/per month, p = 0.036, respectively). While analyzing the temporal SBRT outcomes, the 2 weeks to 3 months period demonstrated the most favorable outcomes. After 6 months, one patient was ICD therapy-free and the remaining patients demonstrated VT episodes.
Conclusions
Our findings showed that the SBRT was associated with a marked reduction in the burden of VT and ICD interventions especially during first 3 months. Although SBRT does not seem to succeed complete termination of VT in long-term period, our findings support the strategy that SBRT can be utilized for immediate antiarrhythmic palliation in critically ill patients with otherwise untreatable refractory VT and electrical storm.
Similar content being viewed by others
1 Introduction
Noninvasive stereotactic body radioablation therapy (SBRT) has emerged as a novel therapeutic option for ventricular tachycardia (VT) refractory or ineligible to antiarrhythmic drugs and catheter-ablation [1,2,3]. Stereotactic radiotherapy is a radiotherapy technique that allows accurate high doses of radiation to be delivered to targeted tissue with minimal exposure to surrounding anatomic structures. In addition to its wide use in the treatment of malignancy, SBRT has recently been introduced with the ability to provide ablative energy noninvasively to arrhythmogenic substrate while reducing damage to normal cardiac tissue nearby and minimizing patients’ procedural risk [4]. Early feasibility trials of single-fraction SBRT, administered in a single 25 Gy dose, revealed encouraging safety and efficacy outcomes in terms of a significant reduction in VT episodes [5, 6].
Despite the growing body of evidence on SBRT cases, there is still debate regarding whether SBRT has a predominant effect in the early or late period after the procedure. In the majority of the trials, VT episodes were observed to be reduced beginning with the first month after SBRT session [2, 7, 8]. On the contrary, recent reports indicated that SBRT’s antiarrhythmic effects were not primarily acute, implying that this technique may have predominantly more delayed/prolonged antiarrhythmic effects [9, 10]. In connection with this issue, another question to be addressed is whether an early blanking phase is required in SBRT studies. Almost half of the studies examined VT episodes with a blanking interval of 6–12 weeks, while the other half did not [4, 8, 10, 11].
On these grounds, we sought to assess the time course of SBRT’s efficacy as well as the value of using a blanking period (BP) following a SBRT session.
2 Material and methods
Consecutive patients presenting to our institution with VT refractory to conventional therapies including antiarrhythmic medications and catheter ablation were eligible for inclusion. Eligible patients were ≥ 18 years old and had (a) ≥ 3 episodes of sustained monomorphic VT in previous 6 months despite at least one catheter ablation procedure, or (b) VT storm (three or more VT episodes in 24 h) despite at least one catheter ablation procedure or (c) have a contraindication or poor candidates to catheter ablation due to clinical risk assessment (such as dual valve replacement) and multiple comorbidities (such as end-stage heart failure or terminal cancer). Therefore, the decision to proceed with SBRT was made on a case-by-case basis involving a multidisciplinary discussion among at least two experienced electrophysiologists and radiation oncologists, nuclear medicine specialists, and radiation physicists. Patients could not have received past radiotherapy to the anticipated treatment field. Patients provided written informed consent after discussion of the risks and benefits of radiation therapy.
2.1 SBRT target planning and treatment
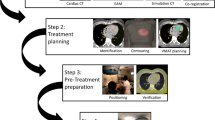
Precise targeting of the arrhythmogenic substrate is paramount to limit adverse effects to healthy myocardium, organs at risk (OAR), and cardiac implantable electronic devices (CIED) [12]. Therefore, the process involves identification of VT substrate/target through a combination of electrophysiological (VT morphologies in ECG and detailed EAM data) and anatomic data (diagnostic imaging by the CT and PET-CT) [13, 14]. The target is referred to as the gross target volume (GTV). Respiratory and cardiac motions have to be incorporated into treatment planning to ensure accurate dose delivery to the target region [15], either through adequate range-considering internal margins or through more conformal strategies such as ECG-based gating or even 4D optimization [16]. Depending on the location (central or peripheral) of the treatment target and treatment purposes, the treatment planning margins for targets and risk volumes should be adjusted accordingly [17]. In this trial, we gave an additional internal margin around the GTV rather than ECG-Gating and 4D optimization to ensure accurate dose delivery to the target for uncertainties that caused by cardiac and respiratory motion as assessed by review of the 4D-CT [6, 13]3, 10. After transferring the CT images on the radiotherapy planning system (Eclipse™, Varian Medical), an average phase CT (avgCT) was created to evaluate cardiac and respiratory movements. All the patients have undergone cardiac PET-CT scan for delineating hypo metabolic target volumes. After fusion of PET-CT and avgCT images using the RT planning software, hypometabolic scar region with the guidance of EAM was created as the internal gross target volume (IGTV). Finally, an additional safety margin of 5 mm was added to the ITV region for treatment planning to create a final planning target volume (PTV), which accounts for any residual uncertainties during positioning, motion, and therapy. Before treatment delivery, re-imaging with the cone beam CT was done for comparing and alignment of images with simulation images. Radiotherapy was given using a linear accelerator device (TrueBeam®, Varian Medical). A total dose of 25 Gy to the PTV in a single fraction to be targeted by at least 95% of the given dose was delivered in 7.5 min [5]. If not contraindicated, oral anticoagulation was prescribed during the first month after treatment. The primary efficacy endpoint was defined as the number of subjects with any reduction in the number of an implantable cardioverter-defibrillator (ICD) treatments for VT episodes and/or any reduction in VT episodes (tracked by indwelling ICD) comparing the 6 months before and after treatment. Therefore, the patients were followed at 1, 3, 6, and 12 months or to the time of death after completion of SBRT.
The radiation therapy–related organ toxicities or adverse events were continuously assessed, and ICD interrogation was performed at each study visit. A pre-specified ICD programming plan was implemented for all patients, which included a zone for detection at least 20 ms slower than the slowest clinical or induced VT. ICD treatments are composed of ICD shocks and anti-tachycardia pacing (ATP). Antiarrhythmic medications were managed at the discretion of the clinician. During follow-up, transthoracic echocardiography was performed at first, third months, and after that 6-month intervals.
2.2 Statistical analysis
Continuous variables with normal distribution were presented as mean (standard deviation [SD]); non-normally distributed variables were reported as median (interquartile range [IQR]). Variables (VT events, ICD shocks, and ATP events) were measured before and after SBRT and compared using the Wilcoxon signed-rank test.
McNemar’s paired testing was used to assess changes in the proportions of anti-arrhythmic use. In the first analysis, the 3 months prior to SBRT and the 3 months post-SBRT were compared for arrhythmia outcomes. In the second analysis, the 3 months prior to SBRT were compared with the average ICD therapies delivered in 3-month windows over total follow-up available [7]. Data were analyzed using SPSS version 21 (IBM Corporation, Armonk, NY). A p-value of < 0.05 was considered statistically significant.
3 Results
Eight patients underwent eight SBRT sessions for refractory VT at our institution between 2019 and 2021 (2 for ischemic cardiomyopathy, 6 for non-ischemic cardiomyopathy including 3 patients with double left-sided mechanical valves, 1 patient with large left ventricular thrombus, 1 patient with dynamic cardiomyopathy, and 1 patient with the burnout stage of hypertrophic cardiomyopathy). Figure 1 shows pre-enrollment endocardial and epicardial ablation images in case 1 with double left-sided mechanical valves. All patients were male with an average age of 58 ± 14 years and a mean ejection fraction of 24 ± 5% (Table 1).
Electrophysiological study and mapping data in case 1. A During electrophysiological study, ventricular tachycardia with the same rate as in intracardiac defibrillator recordings ICD recordings is induced. Twelve lead ECG suggests apical septal inferior exit. B Endocardial map of the left ventricle is seen in left anterior oblique view. The image was obtained using the CARTO® 3 three-dimensional electroanatomic mapping system (Biosense Webster, Diamond Bar, CA, USA) using a transventricular approach in a patient who underwent mechanical double valve replacement. After a mini thoracotomy (C), the three-dimensional electroanatomic maps of epicardium by Decanav® Mapping Catheter (Biosense Webster, Diamond Bar, CA, USA) are created (D). Scar regions (< 0.5 mV) are defined with red color and abnormal electrograms are seen at the bottom of the figure
During the follow-up period, four patients died. Reasons for death were COVID-19 sepsis in two patients, gastric cancer in one patient, and end-stage heart failure with recurrent VT in one patient. One patient (case 1) underwent cardiac transplantation at the 12th months after SBRT session. The workflow for SBRT and tissue level examination findings are showed in Fig. 2.
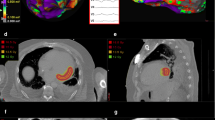
Stereotactic body radioablation therapy workflow and tissue level examination findings in the same patient. Delineation of the internal gross target volume (IGTV) (pink line) and planning target volume (PTV) (red line) that included the full thickness of the myocardium in integrated images from the PET-CT (A) and 4D CBCT (B) using mechanical valves and defibrillator lead for alignment in the axial, coronal, and sagittal views. Apical part of the septum was especially covered because of presumed intramural substrate inaccessible with epicardial approach. Isodose distribution of PTV with delivered dose of 2500 cGy (25 Gy) with doses at least 95% of 25 Gy in the planned target volume and with doses in excess of 25 Gy (110% of 25 Gy) displayed in the color spectrum (C). Device interrogation showing VT episodes before and after SBRT procedure demonstrating no ICD shocks in the first 6 months with following recurrences after this time (red arrow) (D). Macroscopic view of the left ventricle explanted after stereotactic body radiation therapy (E). White areas demonstrate fibrosis from SBRT. Microscopic view of the left ventricle explanted after stereotactic body radiation therapy (F). SBRT causes extensive myocardial interstitial fibrosis with hematoxylin–eosin. Remaining areas demonstrate hypertrophic cardiomyopathy
SBRT was performed on an inpatient basis with the delivery of an average of 25 Gy in a single session. The median noninvasive ablation time was 5.6 min (range, 3.6–7.45 min). The median PTV was 157.4 cc (range, 70.5–272.7 cc) and the median IGTV was 51.7 cc (range, 28.1–116.7 cc). No acute toxicity was observed during or immediately after SBRT. Pericardial effusions were documented in 2 patients: 1 was asymptomatic, 1 resolved with medical management (colchicine and ibuprofen). No definite organ toxicities were detected during follow-up. No adverse outcomes related to ICD pulse generator or lead function were noted in these series.
During a median follow-up of 8 months (range 1 to 14 months), all patients demonstrated VT recurrences; however, ICD shocks and ATP therapies were significantly reduced with SBRT (8.46 to 0.83/per month, p = 0.047; 18.50 to 3.29/per month, p = 0.036, respectively). While analyzing the temporal SBRT outcomes, all patients demonstrated significant reduction in VT episodes and ICD shocks within first 2-week period after SBRT. In this period, only one patient received two ICD shocks. The 2-week to 3-month period demonstrated the most favorable outcomes. There was one patient who had one ICD shock and one patient demonstrated appropriate ATP delivery and NSVT episodes. Six patients were VT-free in this period (Fig. 3). After 3 months, VT recurrences began to occur. In the 3- to 6-month period, 3 patients were shock-free and only two of them were free of any VT episodes. After 6 months, one patient was shock-free and ATP-free, and the remaining three patients demonstrated VT episodes (Table 2).
Implantable cardioverter-defibrillator (ICD) shocks are seen during before and after stereotactic body radiation therapy (SBRT). The line diagram demonstrating the ICD shocks of 8 cases during pre SBRT 3 months and after stereotactic body radiation therapy with follow-up data. Post-SBRT period categorized as first 2 weeks, 2 weeks to 3 months, 3–6 months, and 6–12 months
4 Discussion
Our study demonstrated that SBRT was associated with a marked reduction in the burden of VT and ICD interventions (both shocks and ATP) during the follow-up. SBRT showed the most benefit within 3 months; however, after 6 months, all case showed VT recurrence despite a significant reduction in VT burden and ICD therapies. Additionally, our findings showed that SBRT showed an immediate effect in suppressing VT episodes, questioning the necessity of long blanking periods as 12 weeks. There were few recurrences of VT immediate after the procedure. Moreover, it is worth noting that several treatment areas in this series involved the interventricular septum, which is a difficult or near-impossible area to map with the system. In the light of these findings, regarding its immediate and early VT suppressing effects, SBRT seems to be a viable treatment option for critically ill patients suffering from electrical storm with VT inaccessible to localization and treatment with conventional approaches.
SBRT emerged with the promise to deliver ablative radiation to arrhythmogenic substrate noninvasively while lowering procedural risk and limiting injury to normal cardiac tissue nearby. Scarce but growing evidence showed that SBRT demonstrated favorable efficacy outcomes according to a significant reduction in VT episodes with an acceptable safety profile [2, 5, 7, 10, 18]. However, the findings on whether SBRT has predominantly early or delayed anti-arrhythmic effects are contradictory. The majority of studies claimed that the time course of the decline in VT attacks after SBRT was acute [2, 5, 7]; in contrast, Neuwirth et al. and, more recently [10], Kautzner et al. indicated that SBRT had predominantly delayed effects after 6–12 months [9]. In animal models, SBRT caused dose-dependent myocardial degeneration and subsequent fibrosis progressing from epicardial tissue to full transmurality in the months [19]. Ionizing radiation-induced extensive transmural fibrosis, which eliminates zig-zag conduction in surviving fibers and reduces re-entry, has been proposed as the principal anti-arrhythmic mechanism of action [20]. However, in most studies, post-radioablation fibrosis is not necessarily transmural while surviving fibers might actually be pro-arrhythmic [21]. Notably, in following trials, the effects of radioablation seemed to occur immediately after the procedure implying that the reduction in ventricular arrhythmia burden cannot be attributed solely to scar maturation [3, 22]. SBRT studies evaluating the radiobiological mechanisms of acute cellular injury after SBRT indicated that cell-to-cell conduction disturbances and cellular membrane instability may have an antiarrhythmic impact before the onset of fibrosis [3]. Caspase protein, which is involved in programmed cell death, was found in cells 3 months after radioablation but not 6 months later [23]. Furthermore, three investigations found a substantial upregulation of the gap junction protein, Connexin 43 as early as 2 weeks following therapy, which lasted for up to a year [24,25,26]. After radioablation, upregulation of this protein appeared to be functional, with enhanced conduction velocity and decreased repolarization heterogeneity, which is compatible with upregulation of functional gap junctions [24,25,26]. These findings suggest that radioablation’s acute anti-arrhythmic impact may involve increased conduction due to upregulation of gap junctions in the target area [3]. However, in the recent case report of Benali K. et al., they observed the reduction in bipolar voltages for the myocardium exposed to radiation doses > 15 Gy and suggested that two antiarrhythmic effects of SBRT may coexist: an early impact described by electrical cell reprogramming and a later ablative effect explained by enhanced scar formation [27]. Consistent with these findings, we observed the acute suppression of VT episodes beginning with the immediate after the procedure. Accordingly, the histopathological findings of our patient demonstrated massive fibrosis with rare viable myocytes in the SBRT applied area. This adjacent viable myocyte may be pro-arrhythmic and can be accused of delayed VT recurrences that occurred in this patient.
From another point of view, technical differences in SBRT may be causing the acute or late effects of SBRT to be more pronounced. Since pre-clinical dose studies have shown that doses > 30 Gy to the PTV are required to achieve consistent scar formation at 3–6 months, suboptimal radiation coverage and underdosing of 25 Gy might have led to an initial beneficial effect because of acute edema, but then recurrence of VT in the long term because of the lack of uniform myocardial cell death and/or higher arrhythmic risk of terminally differentiated cells [2].
The requirement for BP and the uniform delivery of 25 Gy regardless of scar size or characteristics requires further investigation. Additionally, it is worth pointing out that the studies speculating predominantly delayed efficacy of SBRT used a different platform as the robotic treatment system CyberKnife delivering the single dose of 25 Gy to the smaller targeted area resulting more concentrating radiation [9, 10]. Considering these previous studies, we assessed that more focused energy may lead to success more transmural scarring and less tissue-related changes in membrane disturbances resulting in more delayed effects rather than acute impacts. However, there is no information on potential differences about histologic changes in the tissue between different platforms.
The other issue addressed in our study is the necessity and duration of blanking period after SBRT session. Some studies examined the SBRT results after a blanking interval of 2–12 weeks; however, the others did not determine a blanking period. Robinson et al. reported that VT episodes were significantly reduced in the first 6 weeks [8]. Similarly, Lloyd et al. reported that the time course of SBRT action was mainly immediate, with most patients responding in the first 2 weeks after therapy [7]. Consistent with these studies, in our study, we assessed the VT episodes starting with the SBRT session specifying the first 2 weeks. During the first 2 weeks, we observed ICD shocks in one patient; the rest of the patients were shock-free. The period between 2 weeks and 3 months was the time course when VT episodes occurred the least. We proposed that blanking period is not solely necessary in assessment of SBRT results, and if blanking period is planning to apply, it should not exceed 2 weeks. Otherwise, the early effect of SBRT which may be predominantly early could be overlooked.
4.1 Clinical implications
Due to the relatively short and noninvasive nature of the treatment, this therapy holds promise as a safe and bail-out choice for advanced heart failure patients with electrical storm who have exhausted for all other options. Because of its capability to administer transmural radiation energy to every ventricular region, SBRT provides the potential to overcome some of the limitations of conventional catheter ablation. Patients who were previously regarded as ineligible for any treatment may now be eligible for a safe and effective choice. However, regarding the high recurrence rate after 6 months of therapy, further investigation is needed on options such as re-administration or dose escalation of SBRT sessions.
4.2 Limitations
This is a small, single-center, non-randomized analysis of an investigational therapy. Therefore, to make a statistical analysis or any statistical conclusions cannot be drawn from these small sample size. The study has a retrospective design reviewing prospectively collected data. The long-term safety of this treatment is not known, and only extrapolations from long-term data from the cancer population might be applied at this time. The noninvasive computed tomography (CT)-based surface electrocardiographic imaging has been proposed in initial reports [2, 17]; however, the availability of ECGI remains limited outside of select institutions and countries (28). Therefore, we used this new modality with the guidance of imaging techniques (such as CT, positron emission tomography, cardiac magnetic resonance imaging) integrated by three-dimensional electro-anatomic mapping (EAM) data in the absence of the ECGI. This might be a limitation of this study. Given the small number of patients who qualify for this invasive treatment based on the refractory symptoms, such a trial should be multicenter in design. The results of these very high-risk patients should not be generalized to younger and healthier VT patients. Also, the high rate of recurrence should be considered especially in patients with a long life expectancy.
5 Conclusions
Our findings showed that the SBRT was associated with a marked reduction in the burden of VT and ICD interventions especially during first 3 months. Although SBRT does not seem to achieve as durable long-term success in VT reduction, our findings support the strategy that SBRT can be utilized for immediate antiarrhythmic palliation in critically ill patients with otherwise refractory VT and electrical storm. SBRT should be kept in mind as a “bail-out” procedure after failed catheter ablation or a bridge therapy until transplantation in these patients.
References
Chiu MH, Mitchell LB, Ploquin N, Faruqi S, Kuriachan VP. Review of stereotactic arrhythmia radioablation therapy for cardiac tachydysrhythmias. CJC Open. 2021;3(3):236–47.
Gianni C, Rivera D, Burkhardt JD, Pollard B, Gardner E, Maguire P, et al. Stereotactic arrhythmia radioablation for refractory scar-related ventricular tachycardia. Heart Rhythm. 2020;17(8):1241–8.
van der Ree MH, Blanck O, Limpens J, Lee CH, Balgobind BV, Dieleman EMT, et al. Cardiac radioablation-a systematic review. Heart Rhythm. 2020;17(8):1381–92.
Loo BW Jr, Soltys SG, Wang L, Lo A, Fahimian BP, Iagaru A, et al. Stereotactic ablative radiotherapy for the treatment of refractory cardiac ventricular arrhythmia. Circ Arrhythm Electrophysiol. 2015;8(3):748–50.
Knutson NC, Samson PP, Hugo GD, Goddu SM, Reynoso FJ, Kavanaugh JA, et al. Radiation therapy workflow and dosimetric analysis from a phase 1/2 trial of noninvasive cardiac radioablation for ventricular tachycardia. Int J Radiat Oncol Biol Phys. 2019;104(5):1114–23.
Schmitt D, Blanck O, Gauer T, Fix MK, Brunner TB, Fleckenstein J, et al. Technological quality requirements for stereotactic radiotherapy: expert review group consensus from the DGMP Working Group for Physics and Technology in Stereotactic Radiotherapy. Strahlenther Onkol. 2020;196(5):421–43.
Lloyd MS, Wight J, Schneider F, Hoskins M, Attia T, Escott C, et al. Clinical experience of stereotactic body radiation for refractory ventricular tachycardia in advanced heart failure patients. Heart Rhythm. 2020;17(3):415–22.
Robinson CG, Samson PP, Moore KMS, Hugo GD, Knutson N, Mutic S, et al. Phase I/II trial of electrophysiology-guided noninvasive cardiac radioablation for ventricular tachycardia. Circulation. 2019;139(3):313–21.
Kautzner J, Jedlickova K, Sramko M, Peichl P, Cvek J, Ing LK, et al. Radiation-induced changes in ventricular myocardium after stereotactic body radiotherapy for recurrent ventricular tachycardia. JACC Clin Electrophysiol. 2021;7(12):1487–92.
Neuwirth R, Cvek J, Knybel L, Jiravsky O, Molenda L, Kodaj M, et al. Stereotactic radiosurgery for ablation of ventricular tachycardia. Europace. 2019;21(7):1088–95.
Cuculich PS, Schill MR, Kashani R, Mutic S, Lang A, Cooper D, et al. Noninvasive cardiac radiation for ablation of ventricular tachycardia. N Engl J Med. 2017;377(24):2325–36.
Hohmann S, Henkenberens C, Zormpas C, Christiansen H, Bauersachs J, Duncker D, et al. A novel open-source software-based high-precision workflow for target definition in cardiac radioablation. J Cardiovasc Electrophysiol. 2020;31(10):2689–95.
Lydiard PS, Blanck O, Hugo G, O’Brien R, Keall P. A review of cardiac radioablation (CR) for arrhythmias: procedures, technology, and future opportunities. Int J Radiat Oncol Biol Phys. 2021;109(3):783–800.
Fahmy TS, Wazni OM, Jaber WA, Walimbe V, Di Biase L, Elayi CS, et al. Integration of positron emission tomography/computed tomography with electroanatomical mapping: a novel approach for ablation of scar-related ventricular tachycardia. Heart Rhythm. 2008;5(11):1538–45.
Poon J, Kohli K, Deyell MW, Schellenberg D, Reinsberg S, Teke T, et al. Technical note: cardiac synchronized volumetric modulated arc therapy for stereotactic arrhythmia radioablation - Proof of principle. Med Phys. 2020;47(8):3567–72.
Graeff C, Bert C. Noninvasive cardiac arrhythmia ablation with particle beams. Med Phys. 2018;45(11):e1024–35.
Ouyang Z, Schoenhagen P, Wazni O, Tchou P, Saliba WI, Suh JH, et al. Analysis of cardiac motion without respiratory motion for cardiac stereotactic body radiation therapy. J Appl Clin Med Phys. 2020;21(10):48–55.
Aras D, Ozturk HF, Ozdemir E, Kervan U, Kara M, Cay S, et al. Use of stereotactic radioablation therapy as a bailout therapy for refractory ventricular tachycardia in a patient with a no-entry left ventricle. J Innov Card Rhythm Manag. 2021;12(9):4671–5.
Krüse JJ, Zurcher C, Strootman EG, Bart CI, Schlagwein N, Leer JW, et al. Structural changes in the auricles of the rat heart after local ionizing irradiation. Radiother Oncol. 2001;58(3):303–11.
de Bakker JM, van Capelle FJ, Janse MJ, van Hemel NM, Hauer RN, Defauw JJ, et al. Macroreentry in the infarcted human heart: the mechanism of ventricular tachycardias with a “focal” activation pattern. J Am Coll Cardiol. 1991;18(4):1005–14.
Talman V, Ruskoaho H. Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell Tissue Res. 2016;365(3):563–81.
Zhang DM, Navara R, Yin T, Szymanski J, Goldsztejn U, Kenkel C, et al. Cardiac radiotherapy induces electrical conduction reprogramming in the absence of transmural fibrosis. Nat Commun. 2021;12(1):5558.
Lehmann HI, Graeff C, Simoniello P, Constantinescu A, Takami M, Lugenbiel P, et al. Feasibility study on cardiac arrhythmia ablation using high-energy heavy ion beams. Sci Rep. 2016;6(1):1–13.
Amino M, Yoshioka K, Tanabe T, Tanaka E, Mori H, Furusawa Y, et al. Heavy ion radiation up-regulates Cx43 and ameliorates arrhythmogenic substrates in hearts after myocardial infarction. Cardiovasc Res. 2006;72(3):412–21.
Amino M, Yoshioka K, Fujibayashi D, Hashida T, Furusawa Y, Zareba W, et al. Year-long upregulation of connexin43 in rabbit hearts by heavy ion irradiation. Am J Physiol Heart Circ Physiol. 2010;298(3):H1014–21.
Amino M, Yoshioka K, Furusawa Y, Tanaka S, Kawabe N, Hashida T, et al. Inducibility of ventricular arrhythmia 1 year following treatment with heavy ion irradiation in dogs with myocardial infarction. Pacing Clin Electrophysiol. 2017;40(4):379–90.
Benali K, Rigal L, Simon A, Bellec J, Jaïs P, Kamakura T, et al. Correlation between the radiation dose and myocardial remodeling after stereotactic radiation therapy for ventricular tachycardia: first assessment of the dose-effect relationship in humans. Heart Rhythm. 2022;S1547–5271(22):01941–5.
Kiani S, Kutob L, Schneider F, Higgins KA, Lloyd MS. Histopathologic and ultrastructural findings in human myocardium after stereotactic body radiation therapy for recalcitrant ventricular tachycardia. Circ Arrhythm Electrophysiol. 2020;13(11): e008753.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Approval was obtained from the local ethics committee.
Informed consent was obtained from all participants.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aras, D., Çetin, E.H.Ö., Ozturk, H.F. et al. Stereotactic body radioablation therapy as an immediate and early term antiarrhythmic palliative therapeutic choice in patients with refractory ventricular tachycardia. J Interv Card Electrophysiol 66, 135–143 (2023). https://doi.org/10.1007/s10840-022-01352-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-022-01352-4