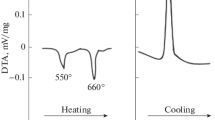
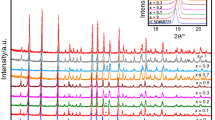
Abstract
Equilibrium phases of the Bi2O3-WO3 system, synthesized using the citrate-gel method with slow cooling, have been systematically investigated for samples with 22–27 mol% WO3. Annealing temperature and content of WO3 were shown to play a significant role in the phases present. Both powder X-ray diffraction and Raman spectroscopy revealed that the room temperature equilibrium phases were 7Bi2O3-WO3 and 7Bi2O3-2WO3, with the latter being dominant. Variable temperature Raman spectroscopy showed that both these phases were present up to 850 °C, with the possible formation of a third unidentified phase at higher temperatures. The ionic conductivities of the mixed-phase materials were between that for the pure 7Bi2O3-WO3 and 7Bi2O3-2WO3 phases and at 700 °C was only about three times lower than that of the pure defect fluorite phase of the 22 mol% WO3 samples. The Arrhenius plots showed no sudden increase in conductivity between 300 and 750 °C providing evidence that no major phase change occurred in this temperature range.







Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
S.P. Adhikari, H. Dean, Z.D. Hood, R. Peng, K.L. More, I. Ivanov, Z. Wu, A. Lachgar, Visible-light-driven Bi2O3/WO3 composites with enhanced photocatalytic activity. RSC Adv. 5(111), 91094–91102 (2015)
X.F. Cao, L. Zhang, X.T. Chen, Z.L. Xue, Microwave-assisted solution-phase preparation of flower-like Bi2WO6 and its visible-light-driven photocatalytic properties. CrystEngComm 13(1), 306–311 (2011)
A.M. Azad, S. Larose, S.A. Akbar, Bismuth oxide-based solid electrolytes for fuel cells. J. Mater. Sci. 29(16), 4135–4151 (1994)
T. Takahashi, H. Iwahara, High oxide ion conduction in sintered oxides of the system Bi2O3 -WO3. J. Appl. Electrochem. 3(1), 65–72 (1973)
T. Takahashi, H. Iwahara, Oxide ion conductors based on bismuth sesquioxide. Mater. Res. Bull 13(12), 1447–1453 (1978)
P. Shuk, H.D. Wiemhöfer, U. Guth, W. Göpel, M. Greenblatt, Oxide ion conducting solid electrolytes based on Bi2O3. Solid State Ion. 89(3–4), 179–196 (1996)
A. Laarif, F. Theobald, The lone pair concept and the conductivity of bismuth oxides Bi2O3. Solid State Ion. 21(3), 183–193 (1986)
J.C. Boivin, Structural and electrochemical features of fast oxide ion conductors. Int. J. Inorg. Mater. 3(8), 1261–1266 (2001)
C.R. Rao, G.S. Rao, S. Ramdas, Phase transformations and electrical properties of bismuth sesquioxide. J. Phys. Chem. 73(3), 672–675 (1969)
M. Drache, P. Roussel, J.P. Wignacourt, Structures and oxide mobility in Bi – Ln – O materials: heritage of Bi2O3. Chem. Rev. 107(1), 80–96 (2007)
E.M. Levin, R.S. Roth, Polymorphism of bismuth sesquioxide. II. Effect of oxide additions on the polymorphism of Bi2O3. J. Res. Natl Inst. Stand. Technol. 68(2), 197–206 (1964)
N. Jiang, E.D. Wachsman, S.H. Jung, A higher conductivity Bi2O3-based electrolyte. Solid State Ion. 150(3–4), 347–353 (2002)
A. Watanabe, M. Sekita, Stabilized δ-Bi2O3 phase in the system Bi2O3–Er2O3–WO3 and its oxide-ion conduction. Solid State Ion. 176(31–34), 2429–2433 (2005)
D. Mercurio, M.El Farissi, B. Frit, J.M. Reau, J. Senegas, Fast ionic conduction in new oxide materials of the Bi2O3–Ln2O3–TeO2 systems (Ln = La, Sm, Gd, Er). Solid State Ion. 39(3–4), 297–304 (1990)
N. Portefaix, P. Conflant, J.C. Boivin, J.P. Wignacourt, M. Drache, New Bi–Ln–V–O anionic conductors with δ-Bi2O3 fluorite-type structure (Ln = Y, Sm, Eu, Gd, Tb, Dy, Er, Yb). J. Solid State Chem. 134(2), 219–226 (1997)
K. Shitara, T. Moriasa, A. Sumitani, A. Seko, H. Hayashi, Y. Koyama, R. Huang, D. Han, H. Moriwake, I. Tanaka, First-principles selection of solute elements for Er-stabilized Bi2O3 oxide-ion conductor with improved long-term stability at moderate temperatures. Chem. Mater. 29(8), 3763–3768 (2017)
S. Bandyopadhyay, A. Dutta, A structural insight into the electrical properties of Dy-Ho co-doped phase stabilized Bismuth Oxide based electrolytes. J. Electroanal. Chem. 817, 55–64 (2018)
E.D. Wachsman, K.T. Lee, Lowering the temperature of solid oxide fuel cells. Science 334(6058), 935–939 (2011)
A. Watanabe, Polymorphic transformation of δ-Bi2O3 stabilized with Ln2O3 (Ln = Sm, Eu, Gd, Tb and Dy) into a new phase with a C-type rare-earth oxide-related structure. Solid State Ion. 79, 84–88 (1995)
A. Watanabe, A. Ono, Thermostable region of an oxide ion conductor, Bi7WO13.5 (= 7Bi2O3·2WO3), and the solid solubility extension. Solid State Ion. 174(1–4), 15–18 (2004)
A. Borowska-Centkowska, M. Leszczynska, F. Krok, M. Malys, W. Wrobel, S. Hull, I. Abrahams, Local structure and conductivity behavior in Bi7WO13.5. J. Mater. Chem. A 6(13), 5407–5418 (2018)
A. Borowska-Centkowska, F. Krok, I. Abrahams, W. Wrobel, J.R. Dygas, S. Hull, Thermal variation of structure and electrical conductivity in Bi14WO24. Solid State Ion. 202(1), 14–21 (2011)
S. Gražulis, D. Chateigner, R.T. Downs, A.F.T. Yokochi, M. Quirós, L. Lutterotti, E. Manakova, J. Butkus, P. Moeck, A. Le Bail, Crystallography Open Database–an open-access collection of crystal structures. J. Appl. Crystallogr. 42(4), 726–729 (2009)
T.O.P.A.S. Bruker, version 5. Bruker AXS Inc., Madison, Wisconsin, 2014
G. Bergerhoff, I.D. Brown, in Crystallographic databases, ed. by F. By, G. Allen, R. Bergerhoff, Sievers (International Union of Crystallography, Chester, 1987), pp. 77–95
D.W. Jung, J.C. Nino, K.L. Duncan, S.R. Bishop, E.D. Wachsman, Enhanced long-term stability of bismuth oxide-based electrolytes for operation at 500°C. Ionics 16(2), 97–103 (2010)
H. Jung, S.Y. Chung, Absence of distinctively high grain-boundary impedance in polycrystalline cubic bismuth oxide. J. Korean Ceram. Soc. 54(5), 413–421 (2017)
S.N. Hoda, L.L. Chang, Phase relations in the system Bi2O3-WO3. J. Am. Ceram. Soc. 57(7), 323–326 (1974)
A.E. Danks, S.R. Hall, Z.J.M.H. Schnepp, The evolution of ‘sol–gel’chemistry as a technique for materials synthesis. Mater. Horiz. 3(2), 91–112 (2016)
A.R. West, Solid State Chemistry and its Applications (Wiley, Hoboken, 2014)
F.D. Hardcastle, I.E. Wachs, Raman spectroscopy of bismuth tungstates. J. Raman Spectrosc. 26(6), 407–412 (1995)
V.V. Kharton, F.M.B. Marques, A. Atkinson, Transport properties of solid oxide electrolyte ceramics: a brief review. Solid State Ion. 174(1–4), 135–149 (2004)
Acknowledgements
The authors thank the following for financial support for this work: National Research Foundation (Grant Numbers 78555; 99003; 105852), the DST/NRF Centre of Excellence in Strong Materials and the University of the Witwatersrand.
Funding
National Research Foundation (Grant Numbers 78555; 99003; 105852), the DST/NRF Centre of Excellence in Strong Materials and the University of the Witwatersrand.
Author information
Authors and Affiliations
Contributions
(optional: please review the submission guidelines from the journal whether statements are mandatory)
Corresponding author
Ethics declarations
Conflicts of interest/Competing interests
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 1034 kb)
Rights and permissions
About this article
Cite this article
Masina, S.M., Billing, C., Erasmus, R.M. et al. Insights on the phase transitions, stability and conductivity in the Bi2O3-WO3 system. J Electroceram 46, 47–56 (2021). https://doi.org/10.1007/s10832-021-00243-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10832-021-00243-w