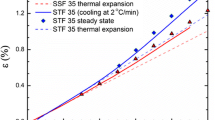
Abstract
Oxygen nonstoichiometry and the defect chemistry of the SrSn1-xFexO3-x/2+δ (SSF) system were examined by means of thermogravimetry as a function of oxygen partial pressure in the temperature range of 700–1000 °C and compared against the corresponding mixed ionic-electronic conducting titanate, SrTi1-xFexO3-x/2+δ (STF) system. The alternate B site host cation, Sn, was selected to replicate and extend the STF studies, given its distinct band structure and higher electron mobility associated with its 5s derived conduction band as compared to the 3d nature of the conduction band in the titanate. Though shifted slightly by the larger size of Sn, the defect equilibria – including the oxygen vacancy concentration – were found to be largely dominated by Fe oxidation state, and thus differed only in a limited way from those in STF. Key thermodynamic parameters for SrSn0.65Fe0.35O2.825+δ (SSF35) were derived including the reduction enthalpy (4.137 ± 0.175 eV), the high temperature electronic band gap (1.755 ± 0.015 eV) and the anion Frenkel enthalpy (0.350 ± 0.350 eV). The implications these observations have for cathode behavior in solid oxide fuel cells are briefly discussed.





Similar content being viewed by others
References
A. Rothschild, W. Menesklou, H.L. Tuller, E. Ivers-Tiffée, Electronic structure, defect chemistry, and transport properties of SrTi1-xFexO3-y solid solutions. Chem. Mater. 18(16), 3651–3659 (2006)
W. Jung, H.L. Tuller, A new model describing solid oxide fuel cell cathode kinetics: model thin film SrTi1-xFexO3-δ mixed conducting oxides–a case study. Adv. Energy Mater. 1, 1184–1191 (2011)
J. Januschewsky, M. Ahrens, A. Opitz, F. Kubel, J. Fleig, Optimized La0.6Sr0.4CoO3-δ thin-film electrodes with extremely fast oxygen-reduction kinetics. Adv. Funct. Mater. 19(19), 3151–3156 (2009)
V. Thangadurai, P. Schmid Beurmann, W. Weppner, Mixed oxide ion and electronic conductivity in perovskite-type SrSnO3 by Fe substitution. Mater. Sci. Eng. B 100(1), 18–22 (2003)
V. Thangadurai, R.A. Huggins, W. Weppner, Use of simple ac technique to determine the ionic and electronic conductivities in pure and Fe-substituted SrSnO3 perovskites. J. Power Sources 108(1–2), 64–69 (2002)
M. Kuhn, J.J. Kim, S.R. Bishop, H.L. Tuller, Oxygen nonstoichiometry and defect chemistry of perovskite-structured BaxSr1– xTi1–yFeyO3–y/2+δ solid solutions. Chem. Mater. 25(15), 2970–2975 (2013)
K.S. Roh, K.H. Ryu, C.H. Yo, Nonstoichiometry and physical properties of the SrSn1−xFexO3−ySystem. J. Solid State Chem. 142(2), 288–293 (1999)
R.D. Shannon, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 32(5), 751–767 (1976)
N.H. Perry, J.J. Kim, S.R. Bishop, H.L. Tuller, Strongly coupled thermal and chemical expansion in the perovskite oxide system Sr(Ti,Fe)O3-a. J. Mater. Chem. A 3(7), 3602–3611 (2015)
P. Adler, S. Eriksson, Structural properties, Mössbauer spectra, and magnetism of perovskite-type oxides SrFe1–xTixO3–y. Zeitschrift für Anorg. und Allg. Chemie 626(1), 118–124 (2000)
Acknowledgement
This publication is based on work funded by the Skolkovo Institute of Science and Technology (Skoltech) through the “Center for Research, Education and Innovation for Electrochemical Energy Storage” under contract number 186-MRA. Partial support from the National Science Foundation under award number DMR-1507047 is also acknowledged. Structural characterization of the materials was conducted in the MRSEC Shared Experimental Facilities at MIT, supported by the National Science Foundation under award number DMR-1419807. CSK acknowledges S. Cook for valuable discussions on curve fitting. NHP acknowledges support from I2CNER, funded by the World Premier International Research Center Initiative (WPI), MEXT, Japan.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Given that the predicted values of nonstoichiometry δ were rather insensitive to the equilibrium constant for anion Frenkel disorder, we followed an alternative approach by setting H af and \( {K}_{af}^0 \) for SSF35 equal to that of STF35 in reference [6]. For this routine, data points from all temperatures were fit simultaneously by setting enthalpies and pre-exponential terms as variables, instead of fitting equilibrium constants at each temperature. The resulting enthalpies and pre-exponentials are shown in Appendix Table 4. Note that H red for both fitting procedures (Table 3 and Appendix Table 4) were almost identical (within the error of the value from Table 3), and E g was less than 3% different, indicating insensitivity to the anion Frenkel disorder energetics.
Rights and permissions
About this article
Cite this article
Kim, C.S., Bishop, S.R., Perry, N.H. et al. Electro-chemo-mechanical studies of perovskite-structured mixed ionic-electronic conducting SrSn1-xFexO3-x/2+δpart I: Defect chemistry. J Electroceram 38, 74–80 (2017). https://doi.org/10.1007/s10832-017-0064-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10832-017-0064-3