Abstract
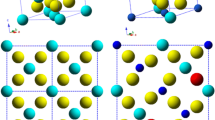
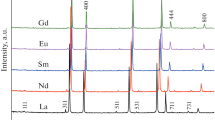
The electrical conductivity of new solid electrolytes Eu2.096Hf1.904O6.952 and Gd2Hf2O7 have been compared with those for different pyrochlores including titanates and zirconates Ln2+xМ2−xO7−δ (Ln = Sm-Lu; M = Ti, Zr; x = 0−0.81). Impedance spectroscopy data demonstrate that Eu2.096Hf1.904O6.952 and Gd2Hf2O7 synthesized from mechanically activated oxides have high ionic conductivity, comparable to that of their zirconate analogues. The bulk and grain-boundary components of conductivity in Sm2.096Hf1.904O6.952 (Тsynth = 1600ºС), Eu2.096Hf1.904O6.952 and Gd2Hf2O7 (Тsynth = 1670ºС) have been determined. The highest bulk conductivity is offered by the disordered pyrochlores prepared at 1600ºC and 1670ºC: ~1.5 × 10−4 S/cm for Sm2.096Hf1.904O6.952, 5 × 10−3 S/cm for Eu2.096Hf1.904O6.952 and 3 × 10−3 S/cm for Gd2Hf2O7 at 780ºС, respectively. The conductivity of the fluorite-like phases at the phase boundaries of the Ln2+xМ2−xO7−δ (Ln = Eu, Gd; M = Zr, Hf; x ~ 0.286) solid solutions, as well as that of the high-temperature fluorite-like phases Ln2+xМ2−xO7−δ (Ln = Eu, Gd; M = Zr, Hf; x = 0−0.286), is lower than the conductivity of the disordered pyrochlores Ln2+xМ2−xO7−δ (Ln = Eu, Gd; M = Zr, Hf; x = 0−0.096).







Similar content being viewed by others
References
M.P. van Dijk, K.J. de Vries, A.J. Burggraaf, Solid State Ion 9/10, 913 (1983). doi:10.1016/0167-2738(83)90110-8
T. van Dijk, K.J. de Vries, A.J. Burggraaf, Phys. Status Solidi (a) 58, 115 (1980). doi:10.1002/pssa.2210580114
T. Takahashi, H. Iwahara, Y. Nagai. J. Appl. Electrochem. 2, 97 (1972). doi:10.1007/BF00609125
V.V. Kharton, A.A. Yaremchenko, E.N. Naumovich, F.M.B. Marques, J. Solid State Electrochem. 4, 243 (2000). doi:10.1007/s100080050202
G.V.M. Kiruthika, K.V. Govindan Kutty, U.V. Varadarju, Solid State Ion. 110, 335 (1998). doi:10.1016/S0167-2738(98)00140-4
B.J. Wuensch, K.W. Eberman, C. Heremans, E.M. Ku, P. Onnerud, E.M.E. Yeo, S.M. Hail, J.K. Stalick, J.D. Jorgensen, Solid State Ion. 129, 111 (2000). doi:10.1016/S0167-2738(99)00320-3
K.J. Moreno, A.F. Fuentes, J. Garcia-Barriocanal, C. Leon, J. Santamaria. J. Solid State Chem. 179, 323 (2006). doi:10.1016/j.jssc.2005.09.036
K.J. Moreno, M.A. Guevara-Liceaga, A.F. Fuentes, J. Garcia-Barriocanal, C. Leon, J. Santamaria. J. Solid State Chem. 179, 928 (2006). doi:10.1016/j.jssc.2005.12.015
A.V. Shlyakhtina, M.V.Boguslavskii Knotko, S.Y. Stefanovich, I.V. Kolbanev, L.L. Larina, L.G. Shcherbakova, Solid State Ion. 178, 59 (2007). doi:10.1016/j.ssi.2006.11.001
P.Y. Butyagin, I.K. Pavlichev, React. Solids 1, 361 (1986). doi:10.1016/0168-7336(86)80027-4
D. Michel, M. Perez, Y. Jorba, R. Collongues, Mater. Res. Bull. 9, 1457 (1974). doi:10.1016/0025-5408(74)90092-0
M.P. van Dijk, F.C. Mijlhoff, A.J. Burggraaf, J. Solid State Chem. 62, 377 (1986). doi:10.1016/0022-4596(86)90253-7
T. Uehara, K. Koto, F. Kanamaru. Solid State Ion. 23, 137 (1987). doi:10.1016/0167-2738(87)90093-2
A.V. Shlyakhtina, D.A. Belov, O.K. Karyagina, L.G. Shcherbakova. J. of Alloys and Compounds, published on line 25.12.2008
H. Takamura, H.L. Tuller, Solid State Ion. 134, 67 (2000). doi:10.1016/S0167-2738(00)00715-3
K.W. Eberman, B.J. Wuensch, J.D. Jorgensen, Solid State Ion. 148, 521 (2002). doi:10.1016/S0167-2738(02)00099-1
A.V. Shlyakhtina, A.V. Knotko, M.V. Boguslavskii, S.Y. Stefanovich, D.V. Peryshkov, I.V. Kolbanev, L.G. Shcherbakova, Solid State Ion. 176, 2297 (2005). doi:10.1016/j.ssi.2005.06.005
A.F. Fuentes, K. Boulahya, M. Maszka, U. Amador. Solid State Sci. 7, 343 (2005). doi:10.1016/j.solidstatesciences.2005.01.002
M. Pruneda, E. Artacho. Phys. Rev. B 72, 08510 (2005). doi:10.1103/PhysRevB.72.085107
Acknowledgements
This work was supported by the Presidium of the Russian Academy of Sciences (program “Synthesis of Inorganic Substances with Controlled Properties and Fabrication of Related Functional Materials,” grant no. 18P/2009), the Russian Foundation for Basic Research (grant no. 07-03-00716), the Department of Materials Sciences of the Russian Academy of Sciences (program of the Basic Investigations of New Metal, Ceramic, Glass- and Composite-Materials and the Deutsche Forschungsgemeinschaft (grant no. CZ 436RUS17/96/06).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shlyakhtina, A.V., Savvin, S.N., Levchenko, A.V. et al. Study of bulk and grain-boundary conductivity of Ln2+xHf2−xO7−δ (Ln = Sm-Gd; x = 0, 0.096) pyrochlores. J Electroceram 24, 300–307 (2010). https://doi.org/10.1007/s10832-009-9572-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10832-009-9572-0