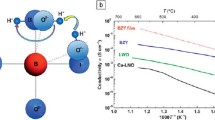
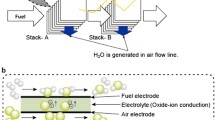
Abstract
This paper outlines the development status, issues, and applications of several solid electrolyte electrochemical devices currently being developed by Ceramatec and its partners. Ceramatec and its commercial partner Air Products and Chemicals, Inc., (APCI) have successfully developed and demonstrated an electrochemical device that utilizes a ceria-based, solid electrolyte to separate oxygen from air [1, 2]. Other oxygen separator projects utilize ion transport membrane(s) (ITM) composed of mixed ionic and electronic conductors to transport oxygen ions across the membrane by means of a pressure differential driving force to generate high purity oxygen or a chemical reaction driving force to produce synthesis gas from methane (ITM Syngas).
Ceramatec, in partnership with SOFCo, demonstrated kilowatt class solid oxide fuel cell (SOFC) stacks operating on a variety of fuels such as pipeline natural gas and reformed diesel. Ceramatec is presently working with Cummins and SOFCo to develop low cost modular fuel cells under the Department of Energy’s Solid-state Energy Conversion Alliance (SECA) initiative. Some of Ceramatec’s other programs are focused on development of gallate electrolyte based fuel cells [3] and metallic bipolar plates [4] for lower temperature operation.
Similar content being viewed by others
References
D.L. Meixner, et al., J. Electrochem. Soc. 149(9), D132 (2002).
P.N. Dyer, et al., Solid State Ionics 134, 21 (2000).
S. Elangovan et al., in Proc. SOFC VIII (The Electrochemical Society, NJ Pennington 2003).
S. Elangovan et al., in Proc. SOFC VIII (The Electrochemical Society, NJPennington 2003).
A.V. Joshi, U.S. Patent 4,879,016, Nov. 7 (1989).
A.V. Joshi, et al., U.S. Patent 5,021,137, June 4 (1991).
J.A. Nachlas, et al., U.S. Patent 5,298,138, March 29 (1994).
J.A. Nachlas, et al., U.S. Patent 5,338,623, Aug. 16 (1994).
D.M. Taylor, et al., U.S. Patent 5,378,345, Jan. 3 (1995).
J.A. Nachlas, et al., U.S. Patent 5,479,700, Jan. 2 (1996).
M.F. Carolan, et al., U.S. Patent 5,750,279, May 12 (1998).
S. Adler, et al., U.S. Patent 5,868,918, Feb. 9 (1999).
S. Adler, et al., U.S. Patent 6,042,703, March 28 (2000).
S. Adler, et al., U.S. Patent 6,117,288, Sept. 12 (2000).
H. Inaba and H. Tagawa, Solid State Ionics 83, 1 (1996).
S.J. Skinner and J.A. Kilner, Materials Today (31-37), March (2003).
S.P.S. Badwal, F.T. Ciacchi, and J. Drennan, Solid State Ionics 121, 253 (1999).
M. Mogensen, N.M. Sammes, and G.A. Tompsett, Solid State Ionics 129, 63 (2000).
J.M. Ralph and J.A., Kilner, in Solid Oxide Fuel Cells V (SOFC V) edited by U. Stimming, S.C. Singhal, H. Tagawa, W. Lehnert, PV 97–40 (The Electrochemical Society Proceedings Series, NJ, Pennington 1997), p. 1021.
D.L. Meixner and R.A. Cutler, Solid State Ionics 146, 273 (2002).
D.L. Meixner and R.A. Cutler, Solid State Ionics 146, 285 (2002).
A.J. Bard and L.R. Faulkner, Electrochemical Methods Fundamentals and Applications (John Wiley and Sons, NY, NY, 1980), p. 91.
T. Ishihara, M. Higuchi, H. Furutani, T. Fukushima, H. Nishiguchi, and Y. Takita, J. Electrochem. Soc. 5, 144 (1997).
K. Huang, M. Feng, and J.B. Goodenough, J. Am. Cer. Soc., 79, 4 (1996).
K. Huang and J.Goodenough, Final Report to EPRI, Report No. TR-108742, Oct. 1997.
J.W. Yan, Z.G. Lu, Y. Jiang, Y.L. Dong, C.Y. Yu, and W.Z. Li, J. Electrochem. Soc. 149(9), A1132 (2002).
S. Balagopal, I. Bay, and S. Elangovan, in Proc. Fifth European SOFC Forum (2002), p. 233.
U.S. Patent 6,265,095—Interconnect for Solid Oxide Fuel Cells.
E. Batawi, W. Glatz, W. Kraussler, M. Janousek, B. Doggwiler, and R. Diethelm, in Proc. SOFC VI, Electrochem. Soc., edited
V.E. Stein and R.E. Richards, 7th Clean Coal Technology Conference, June 21, 1999.
V.E. Stein and R.E. Richards, 16th International Pittsburgh Coal Conference Oct. 11, 1999.
D. Khang, et al. U.S. Patent 5516359, 1996.
R.M. Thorogood, et al., U.S. Patent 5240480, 1993.
S.L. Russek et al., U.S. Patent 5562754, 1996.
S.L. Russek et al., U.S. Patent 5565017, 1996.
D. Khang, et al., U.S. Patent 5657624, 1997.
M.F. Carolan, C.M. Chen, and S.W. Rynders, ACS Spring Meeting, New Orleans, LA, USA, March 26, 2003.
S. Nataraj, R.B. Moore, and S.L. Russek, U.S. Patent 6048472, 2000.
S. Nataraj and S.L. Russek, U.S. Patent 6077323, 2000.
S. Nataraj and S.L. Russek, U.S. Patent 6110979, 2000.
S. Nataraj, P.N. Dyer, and S.L. Russek, U.S. Patent 6114400, 2000.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Joshi, A.V., Steppan, J.J., Taylor, D.M. et al. Solid Electrolyte Materials, Devices, and Applications. J Electroceram 13, 619–625 (2004). https://doi.org/10.1007/s10832-004-5168-x
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10832-004-5168-x