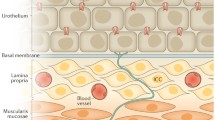
Abstract
The urothelium is the innermost layer of the bladder wall; it plays a pivotal role in bladder sensory transduction by responding to chemical and mechanical stimuli. The urothelium also acts as a physical barrier between urine and the outer layers of the bladder wall. There is intricate sensory communication between the layers of the bladder wall and the neurons that supply the bladder, which eventually translates into the regulation of mechanical activity. In response to natural stimuli, urothelial cells release substances such as ATP, nitric oxide (NO), substance P, acetylcholine (ACh), and adenosine. These act on adjacent urothelial cells, myofibroblasts, and urothelial afferent neurons (UAN), controlling the contractile activity of the bladder. There is rising evidence on the importance of urothelial sensory signalling, yet a comprehensive understanding of the functioning of the urothelium-afferent neurons and the factors that govern it remains elusive to date. Until now, the biophysical studies done on UAN have been unable to provide adequate information on the ion channel composition of the neuron, which is paramount to understanding the electrical functioning of the UAN and, by extension, afferent signalling. To this end, we have attempted to model UAN to decipher the ionic mechanisms underlying the excitability of the UAN. In contrast to previous models, our model was built and validated using morphological and biophysical properties consistent with experimental findings for the UAN. The model included all the channels thus far known to be expressed in UAN, including; voltage-gated sodium and potassium channels, N, L, T, P/Q, R-type calcium channels, large-conductance calcium-dependent potassium (BK) channels, small conductance calcium-dependent (SK) channels, Hyperpolarisation activated cation (HCN) channels, transient receptor potential melastatin (TRPM8), transient receptor potential vanilloid (TRPV1) channel, calcium-activated chloride(CaCC) channels, and internal calcium dynamics. Our UAN model a) was constrained as far as possible by experimental data from the literature for the channels and the spiking activity, b) was validated by reproducing the experimental responses to current-clamp and voltage-clamp protocols c) was used as a base for modelling the non-urothelial afferent neurons (NUAN). Using our models, we also gained insights into the variations in ion channels between UAN and NUAN neurons.






Similar content being viewed by others
Code availability
The source code for the proposed UAN model is available on the GitHub server: https://github.com/sachjoe/BiophysicalmodelUAN.git.
References
Akemann, W., & Knöpfel, T. (2006). Interaction of kv3 potassium channels and resurgent sodium current influences the rate of spontaneous firing of purkinje neurons. Journal of Neuroscience, 26(17), 4602–4612.
Akemann, W., Lundby, A., Mutoh, H., & Knöpfel, T. (2009). Effect of voltage sensitive fluorescent proteins on neuronal excitability. Biophysical Journal, 96(10), 3959–3976.
Amir, R., & Devor, M. (2003a). Electrical excitability of the soma of sensory neurons is required for spike invasion of the soma, but not for through-conduction. Biophysical Journal, 84(4), 2181–2191.
Amir, R., & Devor, M. (2003b). Extra spike formation in sensory neurons and the disruption of afferent spike patterning. Biophysical Journal, 84(4), 2700–2708.
Andersson, K.-E. (2002). Bladder activation: afferent mechanisms. Urology, 59(5), 43–50. https://doi.org/10.1016/s0090-4295(01)01637-5
Andersson, K.-E., & Arner, A. (2004). Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiological Reviews, 84(3), 935–986.
Apodaca, G., Kiss, S., Ruiz, W., Meyers, S., Zeidel, M., & Birder, L. (2003). Disruption of bladder epithelium barrier function after spinal cord injury. American Journal of Physiology-Renal Physiology, 284(5), 966–976. https://doi.org/10.1152/ajprenal.00359.2002
Aruljothi, S., Mandge, D., Manchanda, R. (2017). A biophysical model of heat sensitivity in nociceptive c-fiber neurons. In: 2017 8th International IEEE/EMBS Conference on Neural Engineering (NER), pp. 596–599. IEEE
Baker, M. D. (2005). Protein kinase c mediates up-regulation of tetrodotoxin-resistant, persistent na+ current in rat and mouse sensory neurones. The Journal of Physiology, 567(3), 851–867.
Baumbauer, K. M., DeBerry, J. J., Adelman, P. C., Miller, R. H., Hachisuka, J., Lee, K. H., Ross, S. E., Koerber, H. R., Davis, B. M., & Albers, K. M. (2015). Keratinocytes can modulate and directly initiate nociceptive responses. Elife, 4, 09674.
Birder, L. A., & Groat, W. C. (2007). Mechanisms of disease: involvement of the urothelium in bladder dysfunction. Nature Clinical Practice Urology, 4(1), 46–54. https://doi.org/10.1038/ncpuro0672
Bischoff, U., Vogel, W., & Safronov, B. V. (1998). Na+-activated k+ channels in small dorsal root ganglion neurones of rat. The Journal of Physiology, 510(3), 743–754.
Black, J. A., Cummins, T. R., Yoshimura, N., Groat, W. C., & Waxman, S. G. (2003). Tetrodotoxin-resistant sodium channels nav1. 8/sns and nav1. 9/nan in afferent neurons innervating urinary bladder in control and spinal cord injured rats. Brain Research, 963(1–2), 132–138.
Chambers, J. D., Bornstein, J. C., Gwynne, R. M., Koussoulas, K., & Thomas, E. A. (2014). A detailed, conductance-based computer model of intrinsic sensory neurons of the gastrointestinal tract. American Journal of Physiology-Gastrointestinal and Liver Physiology, 307(5), 517–532.
Choi, J.-S., & Waxman, S. G. (2011). Physiological interactions between nav1. 7 and nav1. 8 sodium channels: a computer simulation study. Journal of Neurophysiology, 106(6), 3173–3184.
DeBerry, J., Albers, K., & Davis, B. (2013). Bladder hypersensitivity and transcriptional regulation of potassium channel subunit mrna expression in mice with cystitis. The Journal of Pain, 14(4), 57.
Du, X., Hao, H., Gigout, S., Huang, D., Yang, Y., Li, L., Wang, C., Sundt, D., Jaffe, D. B., Zhang, H., et al. (2014). Control of somatic membrane potential in nociceptive neurons and its implications for peripheral nociceptive transmission. PAIN®, 155(11), 2306–2322.
Fowler, C. J., Griffiths, D., & De Groat, W. C. (2008). The neural control of micturition. Nature Reviews Neuroscience, 9(6), 453–466.
Fox, A., Nowycky, M., & Tsien, R. (1987). Single-channel recordings of three types of calcium channels in chick sensory neurones. The Journal of Physiology, 394(1), 173–200.
Fukumoto, N., Kitamura, N., Niimi, K., Takahashi, E., Itakura, C., & Shibuya, I. (2012). Ca2+ channel currents in dorsal root ganglion neurons of p/q-type voltage-gated ca2+ channel mutant mouse, rolling mouse nagoya. Neuroscience Research, 73(3), 199–206.
Groat, W. C., Yoshimura, N. (2009). Afferent nerve regulation of bladder function in health and disease. Sensory Nerves, 91–138.
Groat, W. C., Griffiths, D., & Yoshimura, N. (2015). Neural control of the lower urinary tract. Comprehensive Physiology, 5(1), 327.
Han, C., Estacion, M., Huang, J., Vasylyev, D., Zhao, P., Dib-Hajj, S. D., & Waxman, S. G. (2015). Human nav1. 8: enhanced persistent and ramp currents contribute to distinct firing properties of human drg neurons. Journal of Neurophysiology, 113(9), 3172–3185.
Hayashi, Y., Takimoto, K., Chancellor, M. B., Erickson, K. A., Erickson, V. L., Kirimoto, T., Nakano, K., Groat, W. C., & Yoshimura, N. (2009). Bladder hyperactivity and increased excitability of bladder afferent neurons associated with reduced expression of kv1. 4 \(\alpha\)-subunit in rats with cystitis. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 296(5), 1661–1670.
Hilaire, C., Diochot, S., Desmadryl, G., Richard, S., & Valmier, J. (1997). Toxin-resistant calcium currents in embryonic mouse sensory neurons. Neuroscience, 80(1), 267–276.
Hines, M. L., & Carnevale, N. T. (1997). The neuron simulation environment. Neural Computation, 9(6), 1179–1209.
Hodgkin, A. L., & Rushton, W. A. H. (1946). The electrical constants of a crustacean nerve fibre. Proceedings of the Royal Society B, 133, 444–479.
Hougaard, C., Fraser, M., Chien, C., Bookout, A., Katofiasc, M., Jensen, B., Rode, F., Bitsch-Nørhave, J., Teuber, L., Thor, K., et al. (2009). A positive modulator of kca2 and kca3 channels, 4, 5-dichloro-1, 3-diethyl-1, 3-dihydro-benzoimidazol-2-one (ns4591), inhibits bladder afferent firing in vitro and bladder overactivity in vivo. Journal of Pharmacology and Experimental Therapeutics, 328(1), 28–39.
Kanda, H., Clodfelder-Miller, B. J., Gu, J. G., Ness, T. J., & DeBerry, J. J. (2016). Electrophysiological properties of lumbosacral primary afferent neurons innervating urothelial and non-urothelial layers of mouse urinary bladder. Brain Research, 1648, 81–89.
Kovalsky, Y., Amir, R., & Devor, M. (2009). Simulation in sensory neurons reveals a key role for delayed na+ current in subthreshold oscillations and ectopic discharge: implications for neuropathic pain. Journal of Neurophysiology, 102(3), 1430–1442.
Lei, Q., Pan, X.-Q., Villamor, A. N., Asfaw, T. S., Chang, S., Zderic, S. A., & Malykhina, A. P. (2013). Lack of transient receptor potential vanilloid 1 channel modulates the development of neurogenic bladder dysfunction induced by cross-sensitization in afferent pathways. Journal of Neuroinflammation, 10(1), 1–18.
Mandge, D., & Manchanda, R. (2018). A biophysically detailed computational model of bladder small drg neuron soma. PLoS Computational Biology, 14(7), 1006293.
Masoli, S., Solinas, S., & D’Angelo, E. (2015). Action potential processing in a detailed purkinje cell model reveals a critical role for axonal compartmentalization. Frontiers in Cellular Neuroscience, 9, 47.
Matsuyoshi, H., Masuda, N., Chancellor, M. B., Erickson, V. L., Hirao, Y., Groat, W. C., Wanaka, A., & Yoshimura, N. (2006). Expression of hyperpolarization-activated cyclic nucleotide-gated cation channels in rat dorsal root ganglion neurons innervating urinary bladder. Brain Research, 1119(1), 115–123.
Medlock, L., Sekiguchi, K., Hong, S., Dura-Bernal, S., Lytton, W. W., & Prescott, S. A. (2022). Multiscale computer model of the spinal dorsal horn reveals changes in network processing associated with chronic pain. Journal of Neuroscience, 42(15), 3133–3149.
Merrill, L., Gonzalez, E. J., Girard, B. M., & Vizzard, M. A. (2016). Receptors, channels, and signalling in the urothelial sensory system in the bladder. Nature Reviews Urology, 13(4), 193–204.
Nickel, J. C., Jain, P., Shore, N., Anderson, J., Giesing, D., Lee, H., Kim, G., Daniel, K., White, S., Larrivee-Elkins, C., et al. (2012). Continuous intravesical lidocaine treatment for interstitial cystitis/bladder pain syndrome: safety and efficacy of a new drug delivery device. Science Translational Medicine, 4(143), 143–100143100.
Passmore, G. M., Selyanko, A. A., Mistry, M., Al-Qatari, M., Marsh, S. J., Matthews, E. A., Dickenson, A. H., Brown, T. A., Burbidge, S. A., Main, M., et al. (2003). Kcnq/m currents in sensory neurons: significance for pain therapy. Journal of Neuroscience, 23(18), 7227–7236.
Rastogi, P., Rickard, A., Dorokhov, N., Klumpp, D. J., & McHowat, J. (2008). Loss of prostaglandin e2release from immortalized urothelial cells obtained from interstitial cystitis patient bladders. American Journal of Physiology-Renal Physiology, 294(5), 1129–1135. https://doi.org/10.1152/ajprenal.00572.2007
Ritter, A. M., Martin, W. J., & Thorneloe, K. S. (2009). The voltage-gated sodium channel nav1. 9 is required for inflammation-based urinary bladder dysfunction. Neuroscience Letters, 452(1), 28–32.
Rohatgi, A. (2022). Webplotdigitizer: Version 4.6. https://automeris.io/WebPlotDigitizer
Salzer, I., Gantumur, E., Yousuf, A., & Boehm, S. (2016). Control of sensory neuron excitability by serotonin involves 5ht2c receptors and ca2+-activated chloride channels. Neuropharmacology, 110, 277–286.
Schmidt-Hieber, C., & Bischofberger, J. (2010). Fast sodium channel gating supports localized and efficient axonal action potential initiation. Journal of Neuroscience, 30(30), 10233–10242.
Shieh, C.-C., Turner, S., Zhang, X.-F., Milicic, I., Parihar, A., Jinkerson, T., Wilkins, J., Buckner, S., & Gopalakrishnan, M. (2007). A-272651, a nonpeptidic blocker of large-conductance ca2+-activated k+ channels, modulates bladder smooth muscle contractility and neuronal action potentials. British journal of pharmacology, 151(6), 798–806.
Solinas, S., Forti, L., Cesana, E., Mapelli, J., De Schutter, E., & D’Angelo, E. (2007). Computational reconstruction of pacemaking and intrinsic electroresponsiveness in cerebellar golgi cells. Frontiers in Cellular Neuroscience, 1, 2.
Sundt, D., Gamper, N., & Jaffe, D. B. (2015). Spike propagation through the dorsal root ganglia in an unmyelinated sensory neuron: a modeling study. Journal of Neurophysiology, 114(6), 3140–3153.
Takahashi, R., Yoshizawa, T., Yunoki, T., Tyagi, P., Naito, S., De Groat, W. C., & Yoshimura, N. (2013). Hyperexcitability of bladder afferent neurons associated with reduction of kv1. 4 \(\alpha\)-subunit in rats with spinal cord injury. The Journal of Urology, 190(6), 2296–2304.
Tong, W.-C., Choi, C. Y., Karche, S., Holden, A. V., Zhang, H., & Taggart, M. J. (2011). A computational model of the ionic currents, ca 2+ dynamics and action potentials underlying contraction of isolated uterine smooth muscle. PloS One, 6(4), 18685.
Usachev, Y. M., & Thayer, S. A. (1999). Ca2+ influx in resting rat sensory neurones that regulates and is regulated by ryanodine-sensitive ca2+ stores. The Journal of Physiology, 519(1), 115–130.
Yoshimura, N. (1999). Bladder afferent pathway and spinal cord injury: possible mechanisms inducing hyperreflexia of the urinary bladder. Progress in Neurobiology, 57(6), 583–606.
Yoshimura, N., Bennett, N. E., Hayashi, Y., Ogawa, T., Nishizawa, O., Chancellor, M. B., De Groat, W. C., & Seki, S. (2006). Bladder overactivity and hyperexcitability of bladder afferent neurons after intrathecal delivery of nerve growth factor in rats. Journal of Neuroscience, 26(42), 10847–10855.
Yoshimura, N., & Chancellor, M. B. (2003). Neurophysiology of lower urinary tract function and dysfunction. Reviews in Urology, 5(Suppl 8), 3.
Yoshimura, Y., & Yamaguchi, O. (1997). Calcium independent contraction of bladder smooth muscle. International Journal of Urology, 4(1), 62–67. https://doi.org/10.1111/j.1442-2042.1997.tb00142.x
Zemel, B. M., Ritter, D. M., Covarrubias, M., & Muqeem, T. (2018). A-type kv channels in dorsal root ganglion neurons: diversity, function, and dysfunction. Frontiers in Molecular Neuroscience, 11, 253.
Funding
The work was supported by grant from the Department of Biotechnology (DBT), India (BT/PR12973/MED/122/47/2016).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The manuscript does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Action Editor: Upinder Bhalla
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aruljothi, S., Manchanda, R. A biophysically comprehensive model of urothelial afferent neurons: implications for sensory signalling in urinary bladder. J Comput Neurosci 52, 21–37 (2024). https://doi.org/10.1007/s10827-024-00865-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-024-00865-3