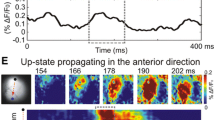
Abstract
The recurrent circuitry of the cerebral cortex generates an emergent pattern of activity that is organized into rhythmic periods of firing and silence referred to as slow oscillations (ca 1 Hz). Slow oscillations not only are dominant during slow wave sleep and deep anesthesia, but also can be generated by the isolated cortical network in vitro, being a sort of default activity of the cortical network. The cortex is densely and reciprocally connected with subcortical structures and, as a result, the slow oscillations in situ are the result of an interplay between cortex and thalamus. Due to this reciprocal connectivity and interplay, the mechanism responsible for the initiation of waves in the corticothalamocortical loop during slow oscillations is still a matter of debate. It was our objective to determine the directionality of the information flow between different layers of the cortex and the connected thalamus during spontaneous activity. With that purpose we obtained multilayer local field potentials from the rat visual cortex and from its connected thalamus, the lateral geniculate nucleus, during deep anaesthesia. We analyzed directionality of information flow between thalamus, cortical infragranular layers (5 and 6) and supragranular layers (2/3) by means of three information theoretical indicators: transfer entropy, symbolic transfer entropy and transcript mutual information. These three indicators coincided in finding that infragranular layers lead the information flow during slow oscillations both towards supragranular layers and towards the thalamus.





Similar content being viewed by others
References
Amigó, J.M. (2010). Permutation complexity in dynamical systems - ordinal patterns, permutation entropy, and all that. Heidelberg: Springer Verlag.
Amigó, J.M. (2012). The equality of kolmogorov-sinai entropy and metric permutation entropy generalized. Physica D, 241, 789–793.
Amigó, J.M., Szczepanski, J., Wajnryb, E., & Sanchez-Vives, M.V. (2004). Estimating the entropy rate of spike trains via lempel-ziv complexity. Neural Computation, 16(4), 717–36.
Amigó, J.M., Zambrano, S., & Sanjuán, M.A.F. (2008). Combinatorial detection of determinism in noisy time series. Europhysics Letters, 83, 60005.
Amigó, J.M., Monetti, R., Aschenbrenner, T., & Bunk, W. (2012). Transcripts: An algebraic approach to coupled time series. Chaos, 22, 013105.
Amigó, J.M., Aschenbrenner, T., Bunk, W., & Monetti, R. (2014). Dimensional reduction of conditional algebraic multi-information via transcripts. Information Sciences, 278, 298–310.
Arnold, M.M., Szczepanski, J., Montejo, N., Amigó, J.M., Wajnryb, E., & Sanchez-Vives, M.V. (2013). Information content in cortical spike trains during brain state transitions. Journal of Sleep Research, 22, 13–21.
Beltramo, R., D’Urso, G., Dal Maschio, M., Farisello, P., Bovetti, S., Clovis, Y., Lassi, G., Tucci, V., De Pietri Tonelli, D., & Fellin, T. (2013). Layer-specific excitatory circuits differentially control recurrent network dynamics in the neocortex. Nature Neuroscience, 16(2), 227–34.
Besserve, M., Schölkopf, B., Logothetis, N., & Panzeri, S. (2010). Causal relationships between frequency bands of extracellular signals in visual cortex revealed by an information theoretic analysis. Journal of Computational Neuroscience, 29(3), 547–566.
Borst, A., & Theunissen, F.E. (1999). Information theory and neural coding. Nature Neuroscience, 2, 947–957.
Chauvette, S., Volgushev, M., & Timofeev, I. (2010). Origin of active states in local neocortical networks during slow sleep oscillation. Cereb Cortex, 20(11), 2660–74.
Compte, A., Sanchez-Vives, M.V., McCormick, D.A., & Wang, X.J. (2003). Cellular and network mechanisms of slow oscillatory activity (<1 hz) and wave propagations in a cortical network model. Journal of Neurophysiology, 89(5), 2707–25.
Crunelli, V., & Hughes, S.W. (2010). The slow (<1 hz) rhythm of non-rem sleep: a dialogue between three cardinal oscillators. Nature Neuroscience, 13(1), 9–17.
Crunelli, V., David, F., Lőrincz, M.L., & Hughes, S.W. (2015). The thalamocortical network as a single slow wave-generating unit. Current opinion in neurobiology, 31, 72–80.
David, F., Schmiedt, J.T., Taylor, H.L., Orban, G., Di Giovanni, G., Uebele, V.N., Renger, J.J., Lambert, R.C., Leresche, N., & Crunelli, V. (2013). Essential thalamic contribution to slow waves of natural sleep. Journal of Neuroscience, 33(50), 19599–610.
Gourévitch, B., & Eggermont, J.J. (2007). Evaluating information transfer between auditory cortical neurons. Journal of Neurophysiology, 97(3), 2533–2543.
Granger, C.W.J. (1969). Investigating causal relations by econometric models and cross-spectral methods. Econometricai, 37(3), 424–438.
Kantz, H., & Schreiber, T. (2000). Nonlinear Time Series Analysis. Cambridge: Cambridge University Press.
Lemieux, M., Chen, J.Y., Lonjers, P., Bazhenov, M., & Timofeev, I. (2014). The impact of cortical deafferentation on the neocortical slow oscillation. The Journal of Neuroscience, 34(16), 5689–5703.
Ma, C., Pan, X., Wang, R., & Sakagami, M. (2013). Estimating causal interaction between prefrontal cortex and striatum by transfer entropy. Cognitive Neurodynamics, 7(3), 253–261.
MacKay, D., & McCulloch, W.S. (1952). The limiting information capacity of a neuronal link. Bulletin of Mathematical Biophysics, 14, 127–135.
Mattia, M., & Sanchez-Vives, M.V. (2012). Exploring the spectrum of dynamical regimes and timescales in spontaneous cortical activity. Cognitive Neurodynamics, 6(3), 239–50.
McCormick, Y., Shu, DAnd, Hasenstaub, A., Sanchez-Vives, M.V., Badoual, M., & Bal, T. (2003). Persistent cortical activity: mechanisms of generation and effects on neuronal excitability. Cereb Cortex, 13(11), 1219–31.
Monetti, R., Bunk, W., Aschenbrenner, T., & Jamitzky, F. (2009). Characterizing synchronization in time series using information measures extracted from symbolic representations. Physical Review E, 79, 046207.
Monetti, R., Amigó, J.M., Aschenbrenner, T., & Bunk, W. (2013a). Permutation complexity of interacting dynamical systems. European Physical Journal Special Topics, 222, 421–436.
Monetti, R., Bunk, W., Aschenbrenner, T., Springer, S., & Amigó, J.M. (2013b). Information directionality in coupled time series using transcripts. Physical Review E, 88(022911).
Paninski, L. (2003). Estimation of entropy and mutual information. Neural Computation, 15, 1191–1253.
Paxinos, G., & Watson, C. (2004). The rat brain in stereotaxic coordinates. London: Elsevier Academic.
Peters, A., Kara, D.A., & Harriman, K.M. (1985). The neuronal composition of area 17 of rat visual cortex: Numerical considerations. The Journal Of Comparative Neurology, 238, 263– 274.
Rigas, P., & Castro-Alamancos, M.A. (2007). Thalamocortical up states: differential effects of intrinsic and extrinsic cortical inputs on persistent activity. Journal Neuroscience, 27(16), 4261– 4272.
Roux, F., Wibral, M., Singer, W., Aru, J., & Uhlhaas, P.J. (2013). The phase of thalamic alpha activity modulates cortical gamma-band activity: evidence from resting-state meg recordings. Journal Neuroscience, 33(45), 17827–17835.
Sakata, S., & Harris, K.D. (2009). Laminar structure of spontaneous and sensory-evoked population activity in auditory cortex. Neuron, 64(3), 404–18.
Sanchez-Vives, M.V., & Mattia, M. (2014). Slow wave activity as the default mode of the cerebral cortex. Archives italiennes de biologie, 152(2/3), 147-155.
Sanchez-Vives, M.V., & McCormick, D.A. (2000). Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nature Neuroscience, 3(10), 1027–34.
Sanchez-Vives, M.V., Descalzo, V.F., Reig, R., Figueroa, N.A., Compte, A., & Gallego, R. (2008). Rhythmic spontaneous activity in the piriform cortex. Cerebral Cortex, 18(5), 1179– 1192.
Schreiber, T. (2000). Measuring information transfer. Physical Review Letters, 85, 461–464.
Staniek, M., & Lehnertz, K. (2008). Symbolic transfer entropy. Physical Review Letters, 100, 158101.
Steriade, M., Nuñez, A., & Amzica, F. (1993a). Intracellular analysis of relations between the slow (<1 hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. Journal Neuroscience, 13(8), 3266–83.
Steriade, M., Contreras, D., Curró Dossi, R., & Nuñez, A. (1993b). The slow (< 1 hz) oscillation in reticular thalamic and thalamocortical neurons: scenario of sleep rhythm generation in interacting thalamic and neocortical networks. Journal Neuroscience, 13(8), 3284–99.
Stroh, A., Adelsberger, H., Groh, A., Rühlmann, C., Fischer, S., Schierloh, A., Deisseroth, K., & Konnerth, A (2013). Making waves: Initiation and propagation of corticothalamic C a 2+ waves in vivo. Neuron, 77(6), 1136–1150.
Timofeev, I., & Steriade, M. (1996). Low-frequency rhythms in the thalamus of intact-cortex and decorticated cats. Journal of Neurophysiology, 76(6), 4152–4168. ISSN 0022-3077.
Timofeev, I., Grenier, F., Bazhenov, M., Sejnowski, T.J., & Steriade, M. (2000). Origin of slow cortical oscillations in deafferented cortical slabs. Cerebral Cortex, 10(12), 1185–1199.
Wester, J.C., & Contreras, D. (2012). Columnar interactions determine horizontal propagation of recurrent network activity in neocortex. The Journal of Neuroscience, 32(16), 5454–5471.
Wiener, N. (1956). Modern mathematics for the engineer. In Beckenbach, E.F. (Ed.) New York: McGraw-Hill.
Acknowledgements
We thank Thomas Aschenbrenner and Wolfram Bunk from Complex Systems Consulting (CSYSC) for their contribution to the development of the code. This work was financially supported by the Spanish Ministerio de Economia y Competitividad, grants MTM2012-31698 to J.M. Amigó and BFU2011- 27094 to M.V. Sanchez-Vives, and by the EU PF7 FET CORTICONIC, contract 600806 to M.V. Sanchez-Vives.
Conflict of interests
The authors declare that they have no conflict of interest
Author information
Authors and Affiliations
Corresponding author
Additional information
Action Editor: Alain Destexhe
Rights and permissions
About this article
Cite this article
Amigó, J.M., Monetti, R., Tort-Colet, N. et al. Infragranular layers lead information flow during slow oscillations according to information directionality indicators. J Comput Neurosci 39, 53–62 (2015). https://doi.org/10.1007/s10827-015-0563-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-015-0563-7