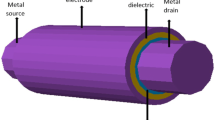
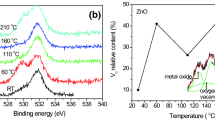
Abstract
In recent years, new emerging oxide materials have demonstrated significant potential for use in gas sensors. We performed a theoretical investigation and analysis of an Ni-doped ZnO (NZO)-based gas sensor. The conductivity and sensitivity of the gas-sensing layer are illustrated as functions of the temperature and gas concentration. The analysis was carried out for an oxidizing agent, i.e., NO2, in which charge is attracted to the adsorbent layer and increases its resistance. The simulation results revealed the variation in resistance versus the temperature and gas concentration. To confirm the feasibility of the model, all the simulation results were compared with reported experimental work. This work will aid researchers in a reasonable choice of materials for and optimal design of high-performance gas sensors.








Similar content being viewed by others
References
Williams, D.E.: Semiconducting oxides as gas-sensitive resistors. Sens. Actuators B Chem. 57, 1–16 (1999)
Jimenez-Cadena, G., Riu, J., Rius, F.X.: Gas sensors based on nanostructured materials. Analyst 132(11), 1083–1099 (2007)
Wang, C., Yin, L., Zhang, L., Xiang, D., Gao, R.: Metal oxide gas sensors: sensitivity and influencing factors. Sensors 10(3), 2088–2106 (2010)
Jagadish, C., Pearton, S.J. (eds.): Zinc Oxide Bulk, Thin Films and Nanostructures: Processing, Properties, and Applications. Elsevier, Amsterdam (2011)
Coleman, V.A., Jagadish, C.: Basic properties and applications of ZnO. In: Zinc Oxide Bulk, Thin Films and Nanostructures: Processing, Properties, and Applications (2006)
Janotti, A., Van de Walle, C.G.: Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 72(12), 126501 (2009)
Fan, Z., Lu, J.G.: Zinc oxide nanostructures: synthesis and properties. J. Nanosci. Nanotechnol. 5(10), 1561–1573 (2005)
Chen, T.-M., Kuschner, W.G., Gokhale, J., Shofer, S.: Outdoor air pollution: nitrogen dioxide, sulfur dioxide, and carbon monoxide health effects. Am. J. Med. Sci. 333(4), 249–256 (2007)
National Research Council: Acute Toxicity of Nitrogen Dioxide (1998)
Ganbavle, V.V., Inamdar, S.I., Agawane, G.L., Kim, J.H., Rajpure, K.Y.: Synthesis of fast response, highly sensitive and selective Ni: ZnO based NO2 sensor. Chem. Eng. J. 286, 36–47 (2016)
Lupan, O., Shishiyanu, S., Chow, L., Shishiyanu, T.: Nanostructured zinc oxide gas sensors by successive ionic layer adsorption and reaction method and rapid photothermal processing. Thin Solid Films 516(10), 3338–3345 (2008)
Xu, M., Li, Q., Ma, Y., Fan, H.: Ni-doped ZnO nanorods gas sensor: enhanced gas-sensing properties, AC and DC electrical behaviors. Sens. Actuators B Chem. 199, 403–409 (2014)
Shirage, P.M., Rana, A.K., Kumar, Y., Sen, S., Leonardi, S.G., Neri, G.: Sr- and Ni-doping in ZnO nanorods synthesized by a simple wet chemical method as excellent materials for CO and CO2 gas sensing. RSC Adv. 6(86), 82733–82742 (2016)
Comsol, A.B.: COMSOL Multiphysics User’s Guide. Version, p. 333 (2005)
Dickinson, E.J.F., Ekström, H., Fontes, Ed: COMSOL multiphysics: finite element software for electrochemical analysis. A mini-review. Electrochem. Commun. 40, 71–74 (2014)
Endres, H.-E., Jander, H.D., Göttler, W.: A test system for gas sensors. Sens. Actuators B Chem. 23(2–3), 163–172 (1995)
Harvey, I., Coles, G., Watson, J.: The development of an environmental chamber for the characterization of gas sensors. Sens. Actuators 16(4), 393–405 (1989)
Niyat, F.Y., Abadi, M.H.S.: COMSOL-based modeling and simulation of SnO2/rGO gas sensor for detection of NO2. Sci. Rep. 8(1), 2149 (2018)
Lazik, D., Sood, P.: Approach for self-calibrating CO2 measurements with linear membrane-based gas sensors. Sensors 16(11), 1930 (2016)
Jaaniso, R., Tan, O.K. (eds.): Semiconductor Gas Sensors. Elsevier, Amsterdam (2013)
Eranna, G.: Metal Oxide Nanostructures as Gas Sensing Devices. CRC Press, Boca Raton (2016)
Trott, G.R., Shorey, A.: Glass wafer mechanical properties: a comparison to silicon. In: 2011 6th International Microsystems, Packaging, Assembly and Circuits Technology Conference (IMPACT), pp. 359–362. IEEE (2011)
Krýsa, J., Novotná, P., Kment, Š., Mills, A.: Effect of glass substrate and deposition technique on the properties of sol gel TiO2 thin films. J. Photochem. Photobiol. A 222(1), 81–86 (2011)
Lee, S., Hinchet, R., Lee, Y., Yang, Y., Lin, Z.-H., Ardila, G., Montès, L., Mouis, M., Wang, Z.L.: Ultrathin nanogenerators as self-powered/active skin sensors for tracking eye ball motion. Adv. Funct. Mater. 24(8), 1163–1168 (2014)
Sun, X.W., Liu, Z.J., Chen, Q.F., Lu, H.W., Song, T., Wang, C.W.: Heat capacity of ZnO with cubic structure at high temperatures. Solid State Commun. 140(5), 219–224 (2006)
Ghosh, C.K., Malkhandi, S., Mitra, M.K., Chattopadhyay, K.K.: Effect of Ni doping on the dielectric constant of ZnO and its frequency dependent exchange interaction. J. Phys. D Appl. Phys. 41(24), 245113 (2008)
Laibinis, P.E., Whitesides, G.M., Allara, D.L., Tao, Y.T., Parikh, A.N., Nuzzo, R.G.: Comparison of the structures and wetting properties of self-assembled monolayers of n-alkanethiols on the coinage metal surfaces, copper, silver, and gold. J. Am. Chem. Soc. 113(19), 7152–7167 (1991)
Mackay, T.G.: On the effective permittivity of silver-insulator nanocomposites. J. Nanophotonics 1(1), 019501 (2007)
Vaartstra, B.A., Marsh, E.P.: Methods for Preparing Ruthenium Oxide Films. U.S. Patent 6,133,159, issued October 17, 2000
Ferizović, D., Hussey, L.K., Huang, Y.-S., Muñoz, M.: Determination of the room temperature thermal conductivity of RuO2 by the photothermal deflection technique. Appl. Phys. Lett. 94(13), 131913 (2009)
Reddy, Y.K.V., Mergel, D.: Structural and electrical properties of RuO2 thin films prepared by rf-magnetron sputtering and annealing at different temperatures. J. Mater. Sci. Mater. Electron. 17(12), 1029–1034 (2006)
Barsan, N., Koziej, D., Weimar, U.: Metal oxide-based gas sensor research: How to? Sens. Actuators B Chem. 121(1), 18–35 (2007)
Clifford, P.K., Tuma, D.T.: Characteristics of semiconductor gas sensors II. Transient response to temperature change. Sens. Actuators 3, 255–281 (1982)
Wang, C.C., Akbar, S.A., Madou, M.J.: Ceramic based resistive sensors. J. Electroceram. 2(4), 273–282 (1998)
Mishra, V.N., Agarwal, R.P.: Sensitivity, response and recovery time of SnO2 based thick-film sensor array for H2, CO, CH4 and LPG. Microelectron. J. 29(11), 861–874 (1998)
Comini, E.: Metal oxide nano-crystals for gas sensing. Anal. Chim. Acta 568(1–2), 28–40 (2006)
Gomri, S., et al.: Adsorption–desorption noise in gas sensors: modelling using Langmuir and Wolkenstein models for adsorption. Sens. Actuators B Chem. 114(1), 451–459 (2006)
Esser, P., Gopel, W.: Physical adsorption on single crystal zinc oxide. Surf. Sci. 97, 309–318 (1980)
Wilson, D.M., Hoyt, S., Janata, J., Booksh, K., Obando, L.: Chemical sensors for portable, handheld field instruments. IEEE Sens. J. 1(4), 256–274 (2001)
Takata, M., Tsubone, D., Yanagida, H.: Dependence of electrical conductivity of ZnO on degree of sensing. J. Am. Ceram. Soc. 59, 4–8 (1976)
Sadek, A.Z., et al.: Characterization of ZnO nanobelt-based gas sensor for H2, NO2, and hydrocarbon sensing. IEEE Sens. J. 7(6), 919–924 (2007)
Stănoiu, A., Simion, C.E., Somăcescu, S.: NO2 sensing mechanism of ZnO–Eu2O3 binary oxide under humid air conditions. Sens. Actuators B Chem. 186, 687–694 (2013)
Saadi, L., Lambert-Mauriat, C., Oison, V., Ouali, H., Hayn, R.: Mechanism of NOx sensing on WO3 surface: first principle calculations. Appl. Surf. Sci. 293, 76–79 (2014)
Caglar, M., Caglar, Y., Ilican, S.: The determination of the thickness and optical constants of the ZnO crystalline thin film by using envelope method. J. Optoelectron. Adv. Mater. 8(4), 1410 (2006)
Srikant, V., Clarke, D.R.: On the optical band gap of zinc oxide. J. Appl. Phys. 83(10), 5447–5451 (1998)
Khemiri, N., et al.: Highly absorbing Cu–In–O thin films for photovoltaic applications. Thin Solid Films 516(20), 7031–7035 (2008)
Peres, N.M.R., Guinea, F., Neto, A.H.C.: Electronic properties of disordered two-dimensional carbon. Phys. Rev. B 73(12), 125411 (2006)
Dingle, R.B., Dingle, R.B.: Asymptotic Expansions: Their Derivation and Interpretation, vol. 48. Academic, London (1973)
Ahmadi, M.T., et al.: Graphene nanoribbon conductance model in parabolic band structure. J. Nanomater. 2010, 12 (2010)
Kažukauskas, V., et al.: Electrical conductivity of carbon nanotubes and polystyrene composites. Phys. Status Solidi (c) 5(9), 3172–3174 (2008)
Areshkin, D.A., Gunlycke, D., White, C.T.: Ballistic transport in graphene nanostrips in the presence of disorder: importance of edge effects. Nano Lett. 7(1), 204–210 (2007)
Ahamdi, M.T., Ismail, R., Anwar, S.: Handbook of Research on Nanoelectronic Sensor Modeling and Applications. IGI Global, USA (2016)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Punetha, D., Dixit, H. & Pandey, S.K. Modeling and analysis of an Ni:ZnO-based Schottky pattern for NO2 detection. J Comput Electron 18, 300–307 (2019). https://doi.org/10.1007/s10825-018-1269-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10825-018-1269-7