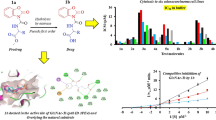
Abstract
Bacterial resistance to the available antibiotic agents underlines an urgent need for the discovery of novel antibacterial agents. Members of the bacterial Mur ligase family MurC–MurF involved in the intracellular stages of the bacterial peptidoglycan biosynthesis have recently emerged as a collection of attractive targets for novel antibacterial drug design. In this study, we have first extended the knowledge of the class of furan-based benzene-1,3-dicarboxylic acid derivatives by first showing a multiple MurC–MurF ligase inhibition for representatives of the extended series of this class. Steady-state kinetics studies on the MurD enzyme were performed for compound 1, suggesting a competitive inhibition with respect to ATP. To the best of our knowledge, compound 1 represents the first ATP-competitive MurD inhibitor reported to date with concurrent multiple inhibition of all four Mur ligases (MurC–MurF). Subsequent molecular dynamic (MD) simulations coupled with interaction energy calculations were performed for two alternative in silico models of compound 1 in the UMA/d-Glu- and ATP-binding sites of MurD, identifying binding in the ATP-binding site as energetically more favorable in comparison to the UMA/d-Glu-binding site, which was in agreement with steady-state kinetic data. In the final stage, based on the obtained MD data novel furan-based benzene monocarboxylic acid derivatives 8–11, exhibiting multiple Mur ligase (MurC–MurF) inhibition with predominantly superior ligase inhibition over the original series, were discovered and for compound 10 it was shown to possess promising antibacterial activity against S. aureus. These compounds represent novel leads that could by further optimization pave the way to novel antibacterial agents.






Similar content being viewed by others
Abbreviations
- ESP:
-
Electrostatic potential
- GAFF:
-
Generalized AMBER Force Field
- LIE:
-
Linear interaction energy method
- LRF:
-
Local reaction field
- MIC:
-
Minimum inhibitory concentration
- RA:
-
Residual activity
- RMSD:
-
Root-mean square distance
- SAR:
-
Structure–activity relationship
- SCAAS:
-
Surface constraint all atoms solvent
- UDP:
-
Uridine-5′-diphosphate
- UMA:
-
Uridine-5′-diphosphate-N-acetylmuramoyl-l-alanine
- UMAG:
-
Uridine-5′-diphosphate-N-acetylmuramoyl-l-alanyl-d-glutamate
- UMT:
-
Uridine-5′-diphosphate-N-acetylmuramoyl-l-alanyl-d-glutamayl-2,6-diaminopimelic acid
References
Silver LL (2006) Does the cell wall of bacteria remain a viable source of targets for novel antibiotics? Biochem Pharmacol 71:996–1005
Brown ED, Wright GD (2005) New targets and screening approaches in antimicrobial drug discovery. Chem Rev 105:759–774
Silver LL (2011) Challenges of antibacterial discovery. Clin Microbiol Rev 24:71–109
Vollmer W, Blanot D, de Pedro MA (2008) Peptidoglycan structure and architecture. FEMS Microbiol Rev 32:149–167
Sink R, Barreteau H, Patin D, Mengin-Lecreulx D, Gobec S, Blanot D (2013) MurD enzymes: some recent developments. Biomol Concepts 4:539–546
van Heijenoort J (2001) Recent advances in the formation of the bacterial peptidoglycan monomer unit. Nat Prod Rep 18:503–519
Smith CA (2006) Structure, function and dynamics in the mur family of bacterial cell wall ligases. J Mol Biol 362:640–655
Bertrand JA, Fanchon E, Martin L, Chantalat L, Auger G, Blanot D, van Heijenoort J, Dideberg O (2000) “Open” structures of MurD: domain movements and structural similarities with folylpolyglutamate synthetase. J Mol Biol 301:1257–1266
Perdih A, Kotnik M, Hodoscek M, Solmajer T (2007) Targeted molecular dynamics simulation studies of binding and conformational changes in E. coli MurD. Proteins Struct Funct Bioinf 68:243–254
Perdih A, Solmajer T (2012) MurD ligase from E. coli: c-terminal domain closing motion. Comput Theor Chem 979:73–81
Bertrand JA, Auger G, Martin L, Fanchon E, Blanot D, Le Beller D, van Heijenoort J, Dideberg O (1999) Determination of the MurD mechanism through crystallographic analysis of enzyme complexes. J Mol Biol 289:579–590
Anderson MS, Eveland SS, Onishi H, Pompliano DL (1996) Kinetic mechanism of the Escherichia coli UDPMurNAc-tripeptide d-alanyl-d-alanine-adding enzyme: use of a glutathione S-transferase fusion. Biochemistry 35:16264–16269
Emanuele JJ Jr, Jin H, Yanchunas J Jr, Villafranca JJ (1997) Evaluation of the kinetic mechanism of Escherichia coli uridine diphosphate-N-acetylmuramate: l-alanine ligase. Biochemistry 36:7264–7271
Perdih A, Hodoscek M, Solmajer T (2009) MurD ligase from E. coli: tetrahedral intermediate formation study by hybrid quantum mechanical/molecular mechanical replica path method. Proteins Struct Funct Bioinf 74:744–759
Bouhss A, Dementin S, van Heijenoort J, Parquet C, Blanot D (2002) MurC and MurD synthetases of peptidoglycan biosynthesis: borohydride trapping of acyl-phosphate intermediates. Methods Enzymol 354:189–196
Zoeiby AE, Sanschagrin F, Levesque RC (2002) Structure and function of the Mur enzymes: development of novel Inhibitors. Mol Microbiol 47:1–12
Sosič I, Barreteau H, Simčič M, Sink R, Cesar J, Zega A, Grdadolnik SG, Contreras-Martel C, Dessen A, Amoroso A, Joris B, Blanot D, Gobec S (2011) Second-generation sulphonamide inhibitors of d-glutamic acid-adding enzyme: activity optimisation with conformationally rigid analogues of d-glutamic acid. Eur J Med Chem 46:2880–2894
Tanner ME, Vaganay S, van Heijenoort J, Blanot D (1996) Phosphinate inhibitors of the d-glutamic acid-adding enzyme of peptidoglycan biosynthesis. J Org Chem 61:1756–1760
Kotnik M, Humljan J, Contreras-Martel C, Oblak M, Kristan K, Hervé M, Blanot D, Urleb U, Gobec S, Dessen A, Solmajer T (2007) Structural and functional characterization of enantiomeric glutamic acid derivatives as potential transition state analogue inhibitors of MurD ligase. J Mol Biol 370:107–115
Tomašić T, Sink R, Zidar N, Fic A, Contreras-Martel C, Dessen A, Patin D, Blanot D, Müller-Premru M, Gobec S, Zega A, Kikelj D, Mašič LP (2012) Dual inhibitor of MurD and MurE ligases from Escherichia coli and Staphylococcus aureus. ACS Med Chem Lett 3:626–630
Perdih A, Bren U, Solmajer T (2009) Binding free-energy calculations of N-sulfonyl glutamic acid inhibitors of MurD ligase. J Mol Model 15:983–996
Perdih A, Wolber G, Solmajer T (2013) Molecular dynamics simulation and linear interaction energy study of d-Glu-based inhibitors of the MurD ligase. J Comput Aided Mol Des 27:723–738
Perdih A, Hrast M, Barreteau H, Gobec S, Wolber G, Solmajer T (2014) Inhibitor design strategy based on an enzyme structural flexibility: a case of bacterial MurD ligase. J Chem Inf Model 54:1451–1466
Perdih A, Kovač A, Wolber G, Blanot D, Gobec S, Solmajer T (2009) Discovery of novel benzene 1,3-dicarboxylic acid inhibitors of bacterial MurD and MurE ligases by structure-based virtual screening approach. Bioorg Med Chem Lett 19:2668–2673
McGovern SL, Stoichet BK (2003) Kinase inhibitors: not just for kinases anymore. J Med Chem 46:1478–1483
Catalyst; Accelrys Software: San Diego
Wolber G, Langer T (2005) LigandScout: 3-D pharmacophores derived from protein-bound ligands and their use as virtual screening filters. J Chem Inf Model 45:160–169
Kirchmair J, Markt P, Distinto S, Wolber G, Langer T (2008) Evaluation of the performance of 3D virtual screening protocols: RMSD comparisons, enrichment assessments, and decoy selection—what can we learn from earlier mistakes? J Comput Aided Mol Des 22:213–228
Cereto-Massague A, Guasch L, Valls C, Mulero M, Pujadas G, Garcia-Vallve S (2012) DecoyFinder: an easy-to-use python GUI application for building target-specific decoy sets. Bioinformatics 28:1661–1662
Jones G, Willett P, Glen RC, Leach AR, Taylor R (1997) Development and validation of a genetic algorithm for flexible docking. J Mol Biol 267:727–748
Perdih A, Hrast M, Barreteau H, Gobec S, Wolber G, Solmajer T (2014) Benzene-1,3-dicarboxylic acid 2,5-dimethylpyrrole derivatives as multiple inhibitors of bacterial Mur ligases (MurC–MurF). Bioorg Med Chem 22:4124–4134
Bochner BR, Ames BN (1982) Complete analysis of cellular nucleotides by two-dimensional thin layer chromatography. J Biol Chem 257:9759–9769
Lasko DR, Wang DIC (1996) On-line monitoring of intracellular ATP concentrations in Escherichia coli fermentations. Biotechnol Bioeng 52:364–372
Gribble FM, Loussouarn G, Tucker SJ, Zhao C, Nichols CG, Ashcroft FM (2000) A novel method for measurement of submembrane ATP concentration. J Biol Chem 275:30046–30049
Beis I, Newsholme EA (1975) The contents of adenine nucleotides, phosphagens and some glycolytic intermediates in resting muscles from vertebrates and invertebrates. Biochem J 152:23–32
Marelius J, Kolmodin K, Feierberg I, Åqvist J (1998) A molecular dynamics program for free energy calculations and empirical valence bond simulations in biomolecular systems. J Mol Graph Model 16:213–225
Åqvist J, Medina C, Samuelson JE (1994) A new method for predicting binding affinity in computer-aided drug design. Protein Eng 7:385–391
Åqvist J, Luzhkov VB, Brandsdal BO (2002) Ligand Binding Affinities from MD simulations. Acc Chem Res 35:358–365
Wermuth C-G (ed) (2008) The practice of medicinal chemistry, 3rd edn. Academic Press, New York
Sinko W, Wang Y, Zhu W, Zhang Y, Feixas F, Cox CL, Mitchell DA, Oldfield E, McCammon JA (2014) Undecaprenyl diphosphate synthase inhibitors: antibacterial drug leads. J Med Chem 57:5693–5701
Brvar M, Perdih A, Hodnik V, Renko M, Anderluh G, Jerala R, Solmajer T (2012) In silico discovery and biophysical evaluation of novel 5-(2-hydroxybenzylidene) rhodanine inhibitors of DNA gyrase B. Bioorg Med Chem 20:2572–2580
Xu Y, Brenning B, Clifford A, Vollmer D, Bearss J, Jones C, McCarthy V, Shi C, Wolfe B, Aavula B, Warner S, Bearss DJ, McCullar MV, Schuch R, Pelzek A, Bhaskaran SS, Stebbins CE, Goldberg AR, Fischetti VA, Vankayalapati H (2013) Discovery of novel putative inhibitors of UDP-GlcNAc 2-epimerase as potent antibacterial agents. ACS Med Chem Lett 4:1142–1147
Tomasić T, Masic LP (2009) Rhodanine as a privileged scaffold in drug discovery. Curr Med Chem 16(13):1596–1629
Baell J, Walters MA (2014) Chemistry: chemical con artists foil drug discovery. Nature 513:481–483
Mariner KR, Trowbridge R, Agarwal AK, Miller K, O’Neill AJ, Fishwick CW, Chopra I (2010) Furanyl-rhodanines are unattractive drug candidates for development as inhibitors of bacterial RNA polymerase. Antimicrob Agents Chemother 54:4506–4509
Tomasić T, Masic LP (2012) Rhodanine as a scaffold in drug discovery: a critical review of its biological activities and mechanisms of target modulation. Expert Opin Drug Discov 7:549–560
Ramirez MA, Borja NL (2008) Epalrestat: an aldose reductase inhibitor for the treatment of diabetic neuropathy. Pharmacotherapy 5:646–655
Wolber G, Dornhofer A, Langer T (2006) Efficient overlay of small organic molecules using 3D pharmacophores. J Comput Aided Mol Des 20:773–788
Cornell WD, Cieplak P, Bayly CI, Gould IR, Merz M Jr, Ferguson DM, Spellmeyer DC, Fox T, Caldwell JW, Kollman PA (1995) A second generation force field for the simulation of proteins, nucleic acids, and organic molecules. J Am Chem Soc 117:5179–5197
Gaussian 09, Revision A.1, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian, Inc., Wallingford, CT
Bayly CI, Cieplak P, Cornell WD, Kollman PA (1993) A well-behaved electrostatic potential based method using charge restrains for deriving atomic charges: the RESP model. J Phys Chem 97:10269–10280
Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML (1983) Comparison of simple potential functions for simulating liquid water. J Chem Phys 79:926–935
King G, Warshel A (1989) A surface constrained all-atom solvent model for effective simulations of polar solutions. J Chem Phys 91:3647–3661
Lee FS, Warshel A (1992) A local reaction field method for fast evaluation of long-range electrostatic interactions in molecular simulations. J Chem Phys 97:3100–3107
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38
Auger G, Martin L, Bertrand J, Ferrari P, Fanchon E, Vaganay S, Petillot Y, van Heijenoort J, Blanot D, Dideberg O (1998) Large-scale preparation, purification, and crystallization of UDP-N-acetylmuramoyl-l-alanine: d-glutamate ligase from Escherichia coli. Prot Express Purif 13:23–29
Lanzetta PA, Alvarez LJ, Reinach PS, Candia O (1979) An improved assay for nanomole amounts of inorganic phosphate. Anal Biochem 100:95–97
Acknowledgments
This work was supported by the Ministry of Higher Education, Science and Technology of the Republic of Slovenia Postdoctoral grant number: Z1-4111. The authors would like thank Dr. Jernej Zidar from the Institute of High Performance Computing (IHPC), Singapure and Barbara Pogorelčnik from the National institute of Chemistry, Slovenia, for their technical assistance with MD calculations. Dr. Sandy Favini, Dr. Carlos Contreras-Martel and Dr. Andréa Dessen from the Bacterial Pathogenicity Group at L’Institut de Biologie Structurale (IBS), Grenoble, France are acknowledged and thanked for performing several initial structural experiments on MurD, MurE and MurF enzymes. Dr. Andreja Kovač from the Faculty of Pharmacy, University of Ljubljana is acknowledged for performing initial inhibition assays of some of the novel compounds discussed in this work. Dr. Katja Kristan from Lek Pharmaceuticals is thanked for useful discussions concerning the steady-state kinetics measurements. We are grateful to Dr. Didier Blanot from the Université Paris-Sud, Orsay, France for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (MPG 61967 kb)
Supplementary material 2 (MPG 59716 kb)
Rights and permissions
About this article
Cite this article
Perdih, A., Hrast, M., Pureber, K. et al. Furan-based benzene mono- and dicarboxylic acid derivatives as multiple inhibitors of the bacterial Mur ligases (MurC–MurF): experimental and computational characterization. J Comput Aided Mol Des 29, 541–560 (2015). https://doi.org/10.1007/s10822-015-9843-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-015-9843-6