Abstract
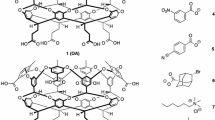
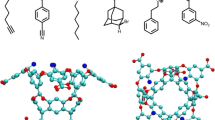
As part of the fourth statistical assessment of modeling of proteins and ligands (sampl.eyesopen.com) prediction challenge, the strength of association of nine guests (1–9) binding to octa-acid host was determined by a combination of 1H NMR and isothermal titration calorimetry. Association constants in sodium tetraborate buffered (pH 9.2) aqueous solution ranged from 5.39 × 102 M−1 in the case of benzoate 1, up to 3.82 × 105 M−1 for trans-4-methylcyclohexanoate 7. Overall, the free energy difference between the free energies of complexation of these weakest and strongest binding guests was ΔΔG° = 3.88 kcal mol−1. Based on a multitude of previous studies, the anticipated order of strength of binding was close to that which was actually obtained. However, the binding of guest 3 (4-ethylbenzoate) was considerably stronger than initially estimated.





Similar content being viewed by others
References
Gibb CLD, Gibb BC (2004) J Am Chem Soc 126:11408
Liu S, Whisenhunt-Ioup SE, Gibb CLD, Gibb BC (2011) Supramol Chem 24:480
The distribution of eight points on the surface of a sphere with the aim of maximizing the distance between them corresponds to a square anti-prism
Gibb CLD, Stevens ED, Gibb BC (2001) J Am Chem Soc 123:5849
Laughrey ZR, Upton T, Gibb BC (2006) Chem Commun 970–972
Liu S, Gibb BC (2008) Chem Commun 3709–3716
Kaanumalle LS, Gibb CLD, Gibb BC, Ramamurthy V (2004) J Am Chem Soc 126:14366
Natarajan A, Kaanumalle LS, Jockusch S, Gibb CLD, Gibb BC, Turro NJ, Ramamurthy V (2007) J Am Chem Soc 129:4132
Gibb CLD, Sundaresan AK, Ramamurthy V, Gibb BC (2008) J Am Chem Soc 130:4069
Gibb CLD, Gibb BC (2006) J Am Chem Soc 128:16498
Liu S, Gan H, Hermann AT, Rick SW, Gibb BC (2010) Nat Chem 2:847
Podkoscielny D, Gibb CLD, Gibb BC, Kaifer AE (2008) Chem Eur J 14:4704
Baldridge A, Samanta SR, Jayaraj N, Ramamurthy V, Tolbert LM (2010) J Am Chem Soc 132:1498
Baldridge A, Samanta SR, Jayaraj N, Ramamurthy V, Tolbert LM (2011) J Am Chem Soc 133(4):712
Porel M, Jockusch S, Parthasarathy A, Rao VJ, Turro NJ, Ramamurthy V (2012) Chem Commun 48(21):2710
Chen JY-C, Jayaraj N, Jockusch S, Ottaviani MF, Ramamurthy V, Turro NJ (2008) J Am Chem Soc 130:7206
Sun H, Gibb CLD, Gibb BC (2008) Supramol Chem 20:141
Gibb CL, Gibb BC (2011) J Am Chem Soc 133:7344–7347
Molecular surface areas, volumes, and dipole moments were calculated using MNDO semi empirical calculations following MMFF molecular mechanics conformer searches. The limited conformational flexibility and high symmetry of the different guests suggest accurate dipole moment calculations using this approach
Mecozzi S, Rebek J Jr (1998) Chem Eur J 4:1016
Gibb CLD, Gibb BC (2011) Thermodynamics of molecular recognition. Supramolecular materials: from molecules to nanomaterials. Wiley, Chichester
The exchange between the free and bound state is very close to the NMR timescale in the case of 7. Thus, although a 2:1 host-guest ratio gave guest signals indicative of slow exchange, this was only because they undergo relatively large shifts from the free state. In contrast, because the host signals undergo only small shifts upon complexation, these signals appeared to undergo fast exchange. As a result, instead of observing both free and complexed host signals, only a time-averaged signal for each proton type was observed (Fig. 3b)
Ewell J, Gibb BC, Rick SW (2008) J Phys Chem B 112:10272
Gibb CLD, Gibb BC (2007) Chem Commun 1635
Gibb CLD, Gibb BC (2009) Tetrahedron 65:7240
Laughrey ZR, Gibb CLD, Senechal T, Gibb BC (2003) Chem Eur J 9:130
Acknowledgments
The authors acknowledge the financial support of the National Institutes of Health (GM098141) in carrying out this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gibb, C.L.D., Gibb, B.C. Binding of cyclic carboxylates to octa-acid deep-cavity cavitand. J Comput Aided Mol Des 28, 319–325 (2014). https://doi.org/10.1007/s10822-013-9690-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-013-9690-2