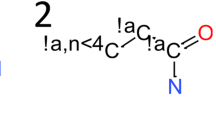Abstract
The Cambridge Structural Database (CSD) offers an excellent data source to study small molecule conformations and molecular interactions. We have analyzed 130 small molecules from the CSD containing an intramolecular sulfur–oxygen distance less than the sum of their van der Waals (vdW) radii. Close S···O distances are observed in several important medicinal chemistry motifs (e.g. a carbonyl oxygen connected by a carbon or nitrogen linker to a sulfur) and are not treated well with existing parameters in the MMFFs or OPLS_2005 force fields, resulting in suboptimal geometries and energetics. In this work, we develop modified parameters for the OPLS_2005 force field to better treat this specific interaction in order to generate conformations close to those found in the CSD structures. We use a combination of refitting a force field torsional parameter, adding a specific atom pair vdW term, and attenuating the electrostatic interactions to obtain an improvement in the accuracy of geometry minimizations and conformational searches for these molecules. Specifically, in a conformational search 58 % of the cases produced a conformation less than 0.25 Å from the CSD crystal conformation with the modified OPLS force field parameters developed in this work. In contrast, 25 and 37 % produced a conformation less than 0.25 Å with the MMFFs and OPLS_2005 force fields, respectively. As an application of the new parameters, we generated conformations for the tyrosine kinase inhibitor axitinib (trade name Inlyta) that could be correctly repacked into three observed polymorphic structures, which was not possible with conformations generated using MMFFs or OPLS_2005. The improved parameters can be mapped directly onto physical characteristics of the systems that are treated inadequately with the molecular mechanics force fields used in this study and potentially other force fields as well.








Similar content being viewed by others
References
Allen F (2002) Acta Crystallogr Sect B 58(3 Part 1):380
Bernstein J (2002) Polymorphism in molecular crystals, vol 14. Oxford University Press, USA
Abramov YA, Pencheva K (2010) Thermodynamics and relative solubility prediction of polymorphic systems. In: am Ende DJ (ed) Chemical engineering in the pharmaceutical industry: R&D to Manufacturing. Wiley, Hoboken, NJ
Kobayashi Y, Ito S, Itai S, Yamamoto K (2000) Int J Pharm 193(2):137
Brittain HG (2009) Polymorphism in pharmaceutical solids. Informa Healthcare, New York
Singhal D, Curatolo W (2004) Adv Drug Deliv Rev 56(3):335
Crowley KJ, Zografi G (2002) J Pharm Sci 91(2):492
Beyer T, Day GM, Price SL (2001) J Am Chem Soc 123(21):5086
Bauer J, Spanton S, Henry R, Quick J, Dziki W, Porter W, Morris J (2001) Pharm Res 18(6):859
Kempf DJ, Marsh KC, Denissen JF, McDonald E, Vasavanonda S, Flentge CA, Green BE, Fino L, Park CH, Kong XP (1995) Proc Nat Acad Sci 92(7):2484
Rascol O, Perez-Lloret S (2009) Expert Opin Pharmacother 10(4):677
Abramov YA, Zell M, Krzyzaniak JF (2010) Toward a rational solvent selection for conformational polymorph screening. In: am Ende DJ (ed) Chemical engineering in the pharmaceutical industry: R&D to manufacturing. Wiley, Hoboken, NJ
Ouvrard C, Price SL (2004) Cryst Growth Des 4(6):1119
Cooper TG, Hejczyk KE, Jones W, Day GM (2008) J Chem Theory Comput 4(10):1795
Day G, Motherwell W, Jones W (2007) Phys Chem Chem Phys 9(14):1693
Iwaoka M, Takemoto S, Okada M, Tomoda S (2002) Bull Chem Soc Jpn 75(7):1611
Burling FT, Goldstein BM (1992) J Am Chem Soc 114(7):2313
Senger S, Chan C, Convery MA, Hubbard JA, Shah GP, Watson NS, Young RJ (2007) Bioorg Med Chem Lett 17(10):2931
Senger S, Convery MA, Chan C, Watson NS (2006) Bioorg Med Chem Lett 16(22):5731
Brameld KA, Kuhn B, Reuter DC, Stahl M (2008) J Chem Inf Model 48(1):1
Reiter LA, Jones CS, Brissette WH, McCurdy SP, Abramov YA, Bordner J, DiCapua FM, Munchhof MJ, Rescek DM, Samardjiev IJ (2008) Bioorg Med Chem Lett 18(9):3000
Kucsman A, Kapovits I (1985) Non-bonded sulfur–oxygen interaction in organic sulfur compounds. In: Bernardi F, Csizmadia IG, Mangini A (eds) Organic sulfur chemistry: theoretical and experimental advances. Elsevier, Amsterdam
Nagao Y, Hirata T, Goto S, Sano S, Kakehi A, Iizuka K, Shiro M (1998) J Am Chem Soc 120(13):3104
Wu S, Greer A (2000) J Org Chem 65(16):4883
Brooks BR, Bruccoleri RE, Olafson BD, States DJ, Swaminathan S, Karplus M (1983) J Comput Chem 4:187
Brooks BR, Brooks C III, Mackerell A Jr, Nilsson L, Petrella R, Roux B, Won Y, Archontis G, Bartels C, Boresch S (2009) J Comp Chem 30(10):1545
Jorgensen WL, Tirado-Rives J (1988) J Am Chem Soc 110:1657
Pranata J, Wierschke SG, Jorgensen WL (1991) J Am Chem Soc 113:2810
Weiner SJ, Kollman PA, Case DA, Singh UC, Ghio C, Alagona G, Profeta J, S., Weiner P (1984) J Am Chem Soc 106:765
Weiner SJ, Kollman PA, Nguyen DT, Case DA (1986) J Comput Chem 7:230
Halgren TA (1992) J Am Chem Soc 114(20):7827
Halgren TA (1996) J Comput Chem 17:520
Halgren TA (1996) J Comput Chem 17:490
Shivakumar D, Harder E, Damm W, Friesner RA, Sherman W (2012) J Chem Theory Comput 8(8):2553
Shivakumar D, Williams J, Wu Y, Damm W, Shelley J, Sherman W (2010) J Chem Theory Comput 6(5):1509
Dauber P, Hagler AT (1980) Acc Chem Res 13(4):105
Brock CP, Minton RP (1989) J Am Chem Soc 111(13):4586
Buntine MA, Hall VJ, Kosovel FJ, Tiekink ERT (1998) J Phys Chem A 102(14):2472
Jorgensen WL, Maxwell DS, Tirado-Rives J (1996) J Am Chem Soc 118(45):11225
Cohen EEW, Rosen LS, Vokes EE, Kies MS, Forastiere AA, Worden FP, Kane MA, Sherman E, Kim S, Bycott P (2008) J Clin Oncol 26(29):4708
Campeta AM, Chekal BP, Abramov YA, Meenan PA, Henson MJ, Shi B, Singer RA, Horspool KR (2010) J Pharm Sci 99(9):3874
Chekal BP, Campeta AM, Abramov YA, Feeder N, Glynn PP, McLaughlin RW, Meenan PA, Singer RA (2009) Org Process Res Dev 13(6):1327
MacKerell AD, Bashford D, Bellott, Dunbrack RL, Evanseck JD, Field MJ, Fischer S, Gao J, Guo H, Ha S, Joseph-McCarthy D, Kuchnir L, Kuczera K, Lau FTK, Mattos C, Michnick S, Ngo T, Nguyen DT, Prodhom B, Reiher WE, Roux B, Schlenkrich M, Smith JC, Stote R, Straub J, Watanabe M, Wiorkiewicz-Kuczera J, Yin D, Karplus M (1998) J Phys Chem B 102(18):3586
Cornell WD, Cieplak P, Bayly CI, Gould IR, Merz KM, Ferguson DM, Spellmeyer DC, Fox T, Caldwell JW, Kollman PA (1995) J Am Chem Soc 117(19):5179
Allinger NL, Yuh YH, Lii JH (1989) J Am Chem Soc 111(23):8551
Schneebeli ST, Bochevarov AD, Friesner RA (2011) J Chem Theory Comput 7(3):658
Mohamadi F, Richards NGJ, Guida WC, Liskamp R, Lipton M, Caufield C, Chang G, Hendrickson T, Still WC (1990) J Comp Chem 11(4):440
Stewart JJP (1989) J Comp Chem 10(2):209
Stewart JJP (1989) J Comp Chem 10(2):221
Speakman JC (1997) Molecular structure by diffraction methods. The Chemical Society, London
Kolossváry I, Guida WC (1996) J Am Chem Soc 118(21):5011
Baker CM, Lopes PEM, Zhu X, Roux B, MacKerell AD (2010) J Chem Theory Comput 6(4):1181
Sun H, Ren P, Fried J (1998) Comp Theor Poly Sci 8(1–2):229
Neumann MA, Perrin MA (2005) J Phys Chem B 109(32):15531
Abramov YA (2011) J Phys Chem A 115(45):12809
Baker RJ, Colavita PE, Murphy DM, Platts JA, Wallis JD (2011) J Phys Chem A 116(5):1435
Jorgensen WL, Schyman P (2012) J Chem Theory Comput. doi:10.1021/ct300180w
Jorgensen WL, Severance DL (1990) J Am Chem Soc 112(12):4768
Acknowledgments
We thank Wolfgang Damm and John Shelley for implementing the NBFIX functionality within the Schrodinger Suite. We also thank Ed Harder for helpful discussions regarding force fields and for comments on the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Lupyan, D., Abramov, Y.A. & Sherman, W. Close intramolecular sulfur–oxygen contacts: modified force field parameters for improved conformation generation. J Comput Aided Mol Des 26, 1195–1205 (2012). https://doi.org/10.1007/s10822-012-9610-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-012-9610-x