Abstract
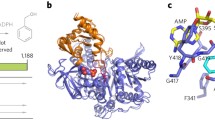
We employed a combination of molecular docking and dynamics to understand the interaction of three different radical scavengers (SB-HSC21, ABNM13 and trimidox) with ribonucleotide reductase M2 (hRRM2) domain. On the basis of the observed results, we can propose how these ligands interact with the enzyme, and cease the radical transfer step from the di-iron center to TYR176. All the ligands alter the electron density over TYR176, –OH group by forming an extremely stable H-bond with either –NHOH group, or with phenolic hydroxyl group of the ligands. This change in electronic density disrupts the water bridge between TYR176, –OH and the di-iron center, which stops the single electron transfer process from TYR176, –OH to iron. As a consequence the enzyme is inhibited. Another interesting observation that we are reporting is the two stage gate keeping mechanism of the RR active site tunnel. We describe these as the outer Gate-1 controlled by ARG330, and the inner Gate-2 controlled by SER263, PHE240, and PHE236. We also observed a dynamic conformational shift in these residues, the incoming ligands can go through, and interact with the underlying TYR176, –OH group. From the study we found the active—site of hRRM2 is extremely flexible and shows a significant induced fit.














Similar content being viewed by others
References
Shao J, Zhou B, Chu B, Yen Y (2006) Ribonucleotide reductase inhibitors and future drug design. Curr Cancer Drug Targets 6:409
Cerqueira NM, Pereira S, Fernandes PA, Ramos MJ (2005) Overview of ribonucleotide reductase inhibitors: an appealing target in anti-tumour therapy. Curr Med Chem 12:1283
Stubbe JA, Nocera DG, Yee CS, Chang MCY (2003) Radical initiation in the class I ribonucleotide reductase: long-range proton-coupled electron transfer? Chem Rev 103:2167
Stubbe JA, van der Donk WA (1998) Protein radicals in enzyme catalysis. Chem Rev 98:705
Jordan A, Reichard P (1998) Ribonucleotide reductases. Annu Rev Biochem 67:71
Reichard P (1993) From RNA to DNA, why so many ribonucleotide reductases? Science 260:1773
Reichard P, Ehrenberg A (1983) Ribonucleotide reductase—a radical enzyme. Science 221:514
Jordan A, Pontis E, Atta M, Krook M, Gibert I, Barbe J, Reichard P (1994) A second class I ribonucleotide reductase in enterobacteriaceae: characterization of the Salmonella typhimurium enzyme. Proc Natl Acad Sci USA 91:12892
Högbom M, Stenmark P, Voevodskaya N, McClarty G, Gräslund A, Nordlund P (2004) The radical site in chlamydial ribonucleotide reductase defines a new R2 subclass. Science 305:245
Smith BD, Karp JE (2003) Ribonucleotide reductase: an old target with new potential. Leuk Res 27:1075
Nocentini G (1996) Ribonucleotide reductase inhibitors: new strategies for cancer chemotherapy. Crit Rev Oncol Hematol 22:89
Holland KP, Elford HL, Bracchi V, Annis CG, Schuster SM, Chakrabarti D (1998) Antimalarial activities of polyhydroxyphenyl and hydroxamic acid derivatives. Antimicrob Agents Chemother 42:2456
Yun D, Saleh L, García-Serres R, Chicalese BM, An YH, Huynh BH, Bollinger JM Jr (2007) Addition of oxygen to the diiron (II/II) cluster is the slowest step in formation of the tyrosyl radical in the W103Y variant of ribonucleotide reductase protein R2 from mouse. Biochemistry 46:13067
Smith P, Zhou B, Ho N, Yuan YC, Su L, Tsai SC, Yen Y (2009) 2.6 Å X-ray crystal structure of human p53R2, a p53-inducible ribonucleotide reductase. Biochemistry 48:11134
Strand KR, Karlsen S, Kolberg M, Røhr ÅK, Görbitz CH, Andersson KK (2004) Crystal structural studies of changes in the native dinuclear iron center of ribonucleotide reductase protein R2 from mouse. J Biol Chem 279:46794
Logan DT, Su XD, Åberg A, Regnström K, Hajdu J, Eklund H, Nordlund P (1996) Crystal structure of reduced protein R2 of ribonucleotide reductase: the structural basis for oxygen activation at a dinuclear iron site. Structure 4:1053
Nordlund P, Sjöberg BM, Eklund H (1990) Three-dimensional structure of the free radical protein of ribonucleotide reductase. Nature 345:593
Nordlund P, Eklund H (1993) Structure and function of the Escherichia coli ribonucleotide reductase protein R2. J Mol Biol 232:123
Končić MZ, Barbarić M, Perković I, Zorc B (2011) Antiradical, chelating and antioxidant activities of hydroxamic acids and hydroxyureas. Molecules 16:6232
Fritzer-Szekeres M, Grusch M, Luxbacher C, Horvath S, Krupitza G, Elford HL, Szekeres T (2000) Trimidox, an inhibitor of ribonucleotide reductase, induces apoptosis and activates caspases in HL-60 promyelocytic leukemia cells. Exp Hematol 28:924
Ren S, Wang R, Komatsu K, Bonaz-Krause P, Zyrianov Y, McKenna CE, Csipke C, Tokes ZA, Lien EJ (2002) Synthesis, biological evaluation, and quantitative structure-activity relationship analysis of new Schiff bases of hydroxysemicarbazide as potential antitumor agents. J Med Chem 45:410
Shao J, Zhou B, Zhu L, Bilio AJ, Su L, Yuan YC, Ren S, Lien EJ, Shih J, Yen Y (2005) Determination of the potency and subunit-selectivity of ribonucleotide reductase inhibitors with a recombinant-holoenzyme-based in vitro assay. Biochem Pharmacol 69:627
Elford HL (1994) assignee. Method of treating hemoglobinopathies. US Patent 5,366,996
Basu A, Sinha BN, Saiko P, Graser G, Szekeres T (2011) N-hydroxy-N′-aminoguanidines as anti-cancer lead molecule: QSAR, synthesis and biological evaluation. Bioorg Med Chem Lett 21:3324
Saiko P, Graser G, Giessrigl B, Lackner A, Grusch M, Krupitza G, Basu A, Sinha B, Jayaprakash V, Jaeger W (2011) A novel N-hydroxy-N′-aminoguanidine derivative inhibits ribonucleotide reductase activity: effects in human HL-60 promyelocytic leukemia cells and synergism with arabinofuranosylcytosine (Ara-C). Biochem Pharmacol 81:50
Krishnan K, Prathiba K, Jayaprakash V, Basu A, Mishra N, Zhou B, Hu S, Yen Y (2008) Synthesis and ribonucleotide reductase inhibitory activity of thiosemicarbazones. Bioorg Med Chem Lett 18:6248
Himo F, Siegbahn PEM (2003) Quantum chemical studies of radical-containing enzymes. Chem Rev 103:2421
Torrent M, Musaev DG, Basch H, Morokuma K (2002) Computational studies of reaction mechanisms of methane monooxygenase and ribonucleotide reductase. J Comput Chem 23:59
Lynch J, Juarez-Garcia C, Münck E, Que L Jr (1989) Mössbauer and EPR studies of the binuclear iron center in ribonucleotide reductase from Escherichia coli. a new iron-to-protein stoichiometry. J Biol Chem 264:8091
Elgren TE, Hendrich MP, Que L Jr (1993) Azide binding to the diferrous clusters of the R2 protein of ribonucleotide reductase from Escherichia coli. J Am Chem Soc 115:9291
Bell CL, Nambury C, Bauer L (1964) The structure of amidoximes. J Org Chem 29:2873
Kjøller Larsen I, Sjöoberg BM, Thelander L (1982) Characterization of the active site of ribonucleotide reductase of Escherichia coli, bacteriophage T4 and mammalian cells by inhibition studies with hydroxyurea analogues. Eur J Biochem 125:75
Acknowledgments
We thank University Grants Commission for providing necessary financial support for the current work. We also thank Mrs. Nibha Mishra and Dr. Venkatesan J for proofreading our manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Basu, A., Sinha, B.N. Understanding the molecular interactions of different radical scavengers with ribonucleotide reductase M2 (hRRM2) domain: opening the gates and gaining access. J Comput Aided Mol Des 26, 865–881 (2012). https://doi.org/10.1007/s10822-012-9581-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-012-9581-y