Abstract
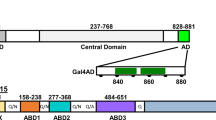
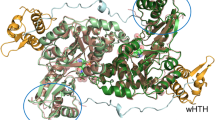
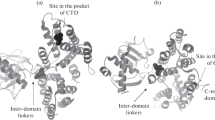
The Gal4p mediated transcriptional activation of GAL genes requires the interaction between Gal3p bound with ATP and galactose and Gal80p. Though numerous studies suggest that galactose and ATP activate Gal3p/Gal1p interaction with Gal80p, neither the mechanism of activation nor the interacting surface that binds to Gal80p is well understood. In this study we investigated the dynamics of Gal3p and Gal1p in the presence and absence of ligands ATP and galactose to understand the role played by dynamics in the function of these proteins through molecular dynamics simulation and protein–protein docking studies. We performed simulations totaling to 510 ns on both Gal1p and Gal3p proteins in the presence and absence of ligands ATP and galactose. We find that, while binding of ligands ATP and galactose to Gal3p/Gal1p do not affect the global conformation of proteins, some local conformational changes around upper-lip helix including insertion domain are observed. We observed that only in the presence of ATP and galactose, Gal3p displays opening and closing motion between the two domains. And because of this motion, a binding interface, which is largely hydrophobic, opens up on the surface of Gal3p and this surface can bind to Gal80p. From our simulation studies we infer probable docking sites for Gal80p on Gal3p/Gal1p, which were further ascertained by the docking of Gal80p on to ligand bound Gal1p and Gal3p proteins, and the residues at the interface between Gal3p and Gal80p are identified. Our results correlate quite well with the existing body of literature on functional and dynamical aspects of Gal1p and Gal3p proteins.











Similar content being viewed by others
References
Suzuki-Fujimoto T, Fukuma M, Yano KI, Sakurai H, Vonika A, Johnston SA, Fukasawa T (1996) Analysis of the galactose signal transduction pathway in Saccharomyces cerevisiae: interaction between Gal3p and Gal80p. Mol Cell Biol 16:2504–2508
Vollenbroich V, Meyer J, Engels R, Cardinali G, Menezes RA, Hollenberg CP (1999) Galactose induction in yeast involves association of Gal80p with Gal1p or Gal3p. Mol Gen Genet 261:495–507
Breunig KD (2000) Regulation of transcription activation by Gal4p. Food Technol Biotechnol 38:287–293
Holden HM, Thoden JB, Timson DJ, Reece RJ (2004) Galactokinase: structure, function and role in type II galactosemia. Cell Mol Life Sci 61:2471–2484
Platt A, Reece RJ (1998) The yeast galactose genetic switch is mediated by the formation of a Gal4p–Gal80p–Gal3p complex. EMBO J 17:4086–4091
Zenke FT, Engels R, Vollenbroich V, Meyer J, Hollenberg CP, Breunig KD (1996) Activation of Gal4p by galactose-dependent interaction of galactokinase and Gal80p. Science 272:1662–1665
Peng G, Hopper JE (2002) Gene activation by interaction of an inhibitor with a cytoplasmic signaling protein. Proc Natl Acad Sci USA 99:8548–8553
Timson DJ, Reece RJ (2002) Kinetic analysis of yeast galactokinase: implications for transcriptional activation of the GAL genes. Biochimie 84:265–272
Timson DJ, Ross HC, Reece RJ (2002) Gal3p and Gal1p interact with the transcriptional repressor Gal80p to form a complex of 1:1 stoichiometry. Biochem J 363:515–520
Jiang F, Frey BR, Evans ML, Friel JC, Hopper JE (2009) Gene activation by dissociation of an inhibitor from a transcriptional activation domain. Mol Cell Biol 29:5604–5610
Peng G, Hopper JE (2000) Evidence for Ga13p’s cytoplasmic location and Ga180p’s dual cytoplasmic-nuclear location implicates new mechanisms for controlling Ga14p activity in Saccharomyces cerevisiae. Mol Cell Biol 20:5140–5148
Bhaumik SR, Raha T, Aiello DP, Green MR (2004) In vivo target of a transcriptional activator revealed by fluorescence resonance energy transfer. Genes Dev 18:333–343
Leuther KK, Johnston SA (1992) Nondissociation of GAL4 and GAL80 in vivo after galactose induction. Science 256:1333–1335
Sil AK, Alam S, Xin P, Ma L, Morgan M, Lebo CM, Woods MP, Hopper JE (1999) The Gal3p–Gal80p–Gal4p transcription switch of yeast: Gal3p destabilizes the Gal80p–Gal4p complex in response to galactose and ATP. Mol Cell Biol 19:7828–7840
Wightman R, Bell R, Reece RJ (2008) Localization and interaction of the proteins constituting the GAL genetic switch in Saccharomyces cerevisiae. Eukaryot Cell 7:2061–2068
Thoden JB, Sellick CA, Timson DJ, Reece RJ, Holden HM (2005) Molecular structure of Saccharomyces cerevisiae Gal1p, a bifunctional galatokinase and transcriptional inducer. J Biol Chem 280:36905–36911
Stagoj MN, Komel R (2008) The GAL induction response in yeasts with impaired galactokinase Gal1p activity. World J Microbiol Biotechnol 24:2159–2166
Sellick CA, Jowitt TA, Reece RJ (2009) The effect of ligand binding on the galactokinase activity of yeast Gal1p and its ability to activate transcription. J Biol Chem 284:229–236
Campbell RN, Leverentz MK, Ryan LA, Reece RJ (2008) Metabolic control of transcription: paradigms and lessons from Saccharomyces cerevisiae. Biochem J 414:177–187
Sellick CA, Campbell RN, Reece RJ (2008) Galactose metabolism in yeast-structure and regulation of the leloir pathway enzymes and the genes encoding them. Int Rev Cell Mol Biol 269:111–150
Melcher K (2005) Mutational hypersensitivity of a gene regulatory protein: Saccharomyces cerevisiae Gal80p. Genetics 171:469–476
Blank TE, Woods MP, Lebo CM, Xin P, Hopper JE (1997) Novel Gal3 proteins showing altered Gal80p binding cause constitutive transcription of Gal4p-activated genes in Saccharomyces cerevisiae. Mol Cell Biol 17:2566–2575
Lavy T, Kumar PR, He H, Joshua-Tor L (2012) The Gal3p transducer of the GAL regulon interacts with the Gal80p repressor in its ligand-induced closed conformation. Genes Dev 26:294–303
Sali A, Blundell TL (1993) Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol 234:779–815
Sanchez R, Sali A (1997) Evaluation of comparative protein structure modeling by MODELLER-3. Proteins 29:50–58
Williams MG, Shirai H, Shi J, Nagendra HG, Mueller J, Mizuguchi K, Miguel RN, Lovell SC, Innis CA, Deane CM et al (2001) Sequence-structure homology recognition by iterative alignment refinement and comparative modeling. Proteins 45:92–97
Melo F, Sanchez R, Sali A (2002) Statistical potentials for fold assessment. Protein Sci 11:430–448
John B, Sali A (2003) Comparative protein structure modeling by iterative alignment, model building and model assessment. Nucleic Acids Res 31:3982–3992
Luthy R, Bowie JU, Eisenberg D (1992) Assessment of protein models with three-dimensional profiles. Nature 356:83–85
Colovos C, Yeates TO (1993) Verification of protein structures: patterns of nonbonded atomic interactions. Protein Sci 2:1511–1519
Laskowski RA, MacArther MW, Moss DS, Thornton JM (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr 26:283–291
Hess B, Kutzner C, Van Der Spoel D, Lindahl E (2008) GRGMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J Chem Theory Comput 4:435–447
Van Gunsteren WF, Billeter SR, Eising AA, Hunenberger PH, Kruger P, Mark AE, Scott WRP, Tironi IG (1996) Biomolecular simulation: the GROMOS96 manual and user guide. Hochschulverlag AG an der ETH Zürich, Zürich, Switzerland, pp 1-1042
Scott WRP, Hanenberger PH, Tironi IG, Mark AE, Billeter SR, Fennen J, Torda AE, Huber T, Krager P, Van Gunsteren WF (1999) The GROMOS biomolecular simulation program package. J Phys Chem A 103:3596–3607
Berendsen HJC, Postma JPM, Van Gunsteren WF, Hermans J (1981) Interaction models for water in relation to protein hydration. In: Pullman B (ed) Intermolecular forces. D. Reidel Publishing Company, Dordrecht, pp 331–342
Schuttelkopf AW, Van Aalten DMF (2004) PRODRG: a tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr D Biol Crystallogr 60:1355–1363
Hess B, Bekker H, Berendsen HJC, Fraaije JGEM (1997) LINCS: a linear constraint solver for molecular simulations. J Comput Chem 18:1463–1472
Essmann U, Perera L, Berkowitz ML, Darden T, Lee H, Pedersen LG (1995) A smooth particle mesh Ewald method. J Chem Phys 103:8577–8593
Berendsen HJC, Postma JPM, Van Gunsteren WF, Dinola A, Haak JR (1984) Molecular dynamics with coupling to an external bath. J Chem Phys 81:3684–3690
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38
Koradi R, Billeter M, Wuthrich K (1996) MOLMOL: a program for display and analysis of macromolecular structures. J Mol Graph 14:51–55
Amadei A, Linssen ABM, Berendsen HJC (1993) Essential dynamics of proteins. Proteins 17:412–425
Amadei A, Linssen ABM, De Groot BL, Berendsen HJC (1995) Essential degrees of freedom of proteins. Mol Eng 5:71–79
Amadei A, Linssen ABM, De Groot BL, Van Aalten DMF, Berendsen HJC (1996) An efficient method for sampling the essential subspace of proteins. J Biomol Struct Dyn 13:615–625
Amadei A, Ceruso MA, Di Nola A (1999) On the convergence of the conformational coordinates basis set obtained by the essential dynamics analysis of proteins’ molecular dynamics simulations. Proteins 36:419–424
Merlino A, Vitagliano L, Ceruso MA, Di Nola A, Mazzarella L (2002) Global and local motions in ribonuclease A: a molecular dynamics study. Biopolymers 65:274–283
De Groot BL, Amadei A, Scheek RM, Van Nuland NAJ, Berendsen HJC (1996) An extended sampling of the configurational space of HPr from E. coli. Proteins 26:314–322
Daura X, Gademann K, Jaun B, Seebach D, Van Gunsteren WF, Mark AE (1999) Peptide folding: when simulation meets experiment. Angew Chem 38:236–240
Dundas J, Ouyang Z, Tseng J, Binkowski A, Turpaz Y, Liang J (2006) CASTp: computed atlas of surface topography of proteins with structural and topographical mapping of functionally annotated residues. Nucleic Acids Res 34:W116–W118
Tirion MM (1996) Large amplitude elastic motions in proteins from a single-parameter, atomic analysis. Phys Rev Lett 77:1905–1908
Lindahl E, Azuara C, Koehl P, Delarue M (2006) NOMAD-Ref: visualization, deformation and refinement of macromolecular structures based on all-atom normal mode analysis. Nucleic Acids Res 34:W52–W56
Tama F, Sanejouand YH (2001) Conformational change of proteins arising from normal mode calculations. Protein Eng 14:1–6
Kumar PR, Yu Y, Sternglanz R, Johnston SA, Joshua-Tor L (2008) NADP regulates the yeast GAL induction system. Science 319:1090–1092
Comeau SR, Gatchell DW, Vajda S, Camacho CJ (2004) ClusPro: an automated docking and discrimination method for the prediction of protein complexes. Bioinformatics 20:45–50
Krissinel E, Henrick K (2007) Inference of macromolecular assemblies from crystalline state. J Mol Biol 372:774–797
Andreassi JL, Vetting MW, Bilder PW, Roderick SL, Leyh TS (2009) Structure of the ternary complex of phosphomevalonate kinase: the enzyme and its family. Biochemistry 48:6461–6468
Thoden JB, Holden HM (2005) The molecular architecture of human N-acetylgalactosamine kinase. J Biol Chem 280:32784–32791
Platt A, Ross HC, Hankin S, Reece RJ (2000) The insertion of two amino acids into a transcriptional inducer converts it into a galactokinase. Proc Natl Acad Sci USA 97:3154–3159
Diep CQ, Peng G, Bewley M, Pilauri V, Ropson I, Hopper JE (2006) Intragenic suppression of Gal3C interaction with Gal80 in the Saccharomyces cerevisiae GAL gene switch. Genetics 172:77–87
Diep CQ, Tao X, Pilauri V, Losiewicz M, Blank TE, Hopper JE (2008) Genetic evidence for sites of interaction between the Gal3 and Gal80 proteins of the Saccharomyces cerevisiae GAL gene switch. Genetics 178:725–736
Pilauri V, Bewley M, Diep C, Hopper J (2005) Gal80 dimerization and the yeast GAL gene switch. Genetics 169:1903–1914
Zhou T, Daugherty M, Grishin NV, Osterman AL, Zhang H (2000) Structure and mechanism of homoserine kinase: prototype for the GHMP kinase superfamily. Structure 8:1247–1257
Krishna SS, Zhou T, Daugherty M, Osterman A, Zhang H (2001) Structural basis for the catalysis and substrate specificity of homoserine kinase. Biochemistry 40:10810–10818
Nogi Y, Fukasawa T (1989) Functional domains of a negative regulatory protein, GAL80, of Saccharomyces cerevisiae. Mol Cell Biol 9:3009–3017
Acknowledgments
S. K. U thanks ICMR (Indian Council of Medical Research) India, for Senior Research Fellowship (Grant No: 3/1/JRF/81/MPD-2006). We thank Prof. P. J. Bhat for introducing us to the biology of GAL genes and appreciate many helpful discussions with him.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Upadhyay, S.K., Sasidhar, Y.U. Molecular simulation and docking studies of Gal1p and Gal3p proteins in the presence and absence of ligands ATP and galactose: implication for transcriptional activation of GAL genes. J Comput Aided Mol Des 26, 847–864 (2012). https://doi.org/10.1007/s10822-012-9579-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-012-9579-5