Abstract
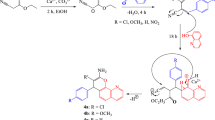
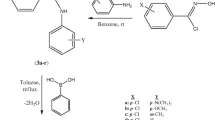
3-Hydroxypyridine-4-one derivatives have shown good inhibitory activity against bacterial strains. In this work we report the application of MOLMAP descriptors based on empirical physicochemical properties with genetic algorithm partial least squares (GA-PLS) and counter propagation artificial neural networks (CP-ANN) methods to propose some novel 3-hydroxypyridine-4-one derivatives with improved antibacterial activity against Staphylococcus aureus. A large collection of 302 novel derivatives of this chemical scaffold was selected for this purpose. The activity classes of these compounds were determined using the two quantitative structure activity relationships models. To evaluate the predictability and accuracy of the obtained models, nineteen compounds belonging to all three activity classes were prepared and the activity of them was determined against S. aureus. Comparing the experimental results and the predicted activity classes revealed the accuracy of the obtained models. Seventeen of the nineteen synthesized molecules were correctly predicted by GA-PLS model according to the antimicrobial evaluation method. Molecules 5f and 5h proved to be moderately active and active experimentally, but were predicted as inactive and moderately active compounds, respectively by this model. The CP-ANN based prediction was correct for sixteen out of the nineteen synthesized molecules. 5a, 5h and 5q were moderately active and active based on the antimicrobial assays, but they were introduced as members of inactive, moderately active and inactive classes of compounds, respectively according to CP-ANN model.






Similar content being viewed by others
Abbreviations
- ANN:
-
Artificial neural networks
- CP-ANN:
-
Counter propagation artificial neural networks
- 2D:
-
Two dimensional
- 3D:
-
Three dimensional
- GA-PLS:
-
Genetic algorithm partial least squares
- MIC:
-
Minimum inhibitory concentration
- MLR:
-
Multiple linear regression
- MOLMAP:
-
Molecular map of atom-level properties
- SOM:
-
Self-organizing maps
- QSAR:
-
Quantitative structure activity relationships
References
Arredondo M, Núñez MT (2005) Iron and copper metabolism. Mol Asp Med 26:313–327
Skaar EP, Gaspar A, Schneewind O (2006) Bacillus anthracis IsdG, a heme-degrading monooxygenase. J Bacteriol 188:1071–1080
Hider R, Kong X (2010) Chemistry and biology of siderophores. Nat Prod Rep 27:637–657
Aytemir M, Erol D, Hider R, Ozalp M (2003) Synthesis and evaluation of antimicrobial activity of new 3-hydroxy-6-methyl-4-oxo-4H-pyran-2-carboxamide derivatives. Turk J Chem 27:757–764
Aytemir M, Hider M, Erol D, Ozalp M, Ekizoglu M (2003) Synthesis of new antimicrobial agents; amide derivatives of pyranones and pyridinones. Turk J Chem 27:445–452
Qiu D, Huang Z, Zhou T, Shen C, Hider R (2011) In vitro inhibition of bacterial growth by iron chelators. FEMS Microbiol Lett 314:107–111
Dehkordi L, Liu Z, Hider R (2008) Basic 3-hydroxypyridin-4-ones: potential antimalarial agents. Eur J Med Chem 43:1035–1047
Kotani T, Ichimoto I, TatsumI C, Fujita T (1975) Structure-activity study of bacteriostatic kojic acid analogs. Agric Biol Chem 39:1311–1317
Kotani T, Ichimoto I, Tatsumi C, Fujita T (1976) Bacteriostatic activities and metal chelation of kojic acid analogs. Agric Biol Chem 40:765–770
Feng M, van der Does L, Bantjes A (1993) Iron (III)-chelating resins. 3. Synthesis, iron (III)-chelating properties, and in vitro antibacterial activity of compounds containing 3-hydroxy-2-methyl-4 (1H)-pyridinone ligands. J Med Chem 36:2822–2827
Fassihi A, Abedi D, Saghaie L, Sabet R, Fazeli H, Bostaki Gh, Deilami O, Sadinpour H (2009) Synthesis, antimicrobial evaluation and QSAR study of some 3-hydroxypyridine-4-one and 3-hydroxypyran-4-one derivatives. Eur J Med Chem 44:2145–2157
Hansch C, Hoekman D, Gao H (1996) Comparative QSAR: toward a deeper understanding of chemicobiological interactions. Chem Rev 96:1045–1075
Horvath D, Mao B (2003) Neighborhood behavior. Fuzzy molecular descriptors and their influence on the relationship between structural similarity and property similarity. QSAR Comb Sci 22:498–509
Putta S, Eksterowicz J, Lemmen C, Stanton R (2003) A novel subshape molecular descriptor. J Chem Inf Comput Sci 43:1623–1635
Ballabio D, Consonni V, Todeschini R (2007) Classification of multiway analytical data based on MOLMAP approach. Anal Chim Acta 605:134–146
Zhang Q, Aires-de-Sousa J (2007) Random forest prediction of mutagenicity from empirical physicochemical descriptors. J Chem Inf Model 47:1–8
Gupta S, Matthew S, Abreu P, Aires-de-Sousa J (2006) QSAR analysis of phenolic antioxidants using MOLMAP descriptors of local properties. Bioorg Med Chem 14:1199–1206
Zupan J, Gasteiger J (1999) Neural networks in chemistry and drug design, 2nd edn. Wiley-VCH, Weinheim
Simon V, Gasteiger J, Zupan J (1993) A combined application of 2 different neural-network types for the prediction of chemical-reactivity. J Am Chem Soc 115:9148–9159
Program PETRA can be tested on the website http://www2.chemie.uni-erlangen.de/software/petra and is developed by Molecular Networks GmbH,http:www.mol-net.de
Zhang Q, Aires-de-Sousa J (2005) Structure-based classification of chemical reactions without assignment of reaction centers. J Chem Inf Model 45:1775–1783
Sabet R, Fassihi A (2008) QSAR study of antimicrobial 3-hydroxypyridine-4-one and 3-hydroxypyran-4-one derivatives using different chemometric tools. Int J Mol Sci 9:2407–2423
Loncle C, Brunel J, Vidal N, Dherbomez M, Letourneux Y (2004) Synthesis and antifungal activity of cholesterol-hydrazone derivatives. Eur J Med Chem 39:1067–1071
Melnyk P, Leroux V, Sergheraert C, Grellier P (2006) Design, synthesis and in vitro antimalarial activity of an acylhydrazone library. Bioorg Med Chem Lett 16:31–35
Kuecuekguezel S, Rollas S, Kuecuekguezel I, Kiraz M (1999) Synthesis and antimycobacterial activity of some coupling products from 4-aminobenzoic acid hydrazones. Eur J Med Chem 34:1093–1100
Dhar D, Taploo C (1982) Schiff bases and their applications. J Sci Ind Res 41:501–504
Przybylski P, Huczynski A, Pyta K, Brzezinski B, Bartl F (2009) Biological properties of schiff bases and azo derivatives of phenols. Curr Org Chem 13:124–148
Todeschini R (2010) Milano Chemometrics and QSPR Group. http://michem.disat.unimib.it/. Accessed 14 March 2010
Haaland D, Thomas E (1988) Partial least-squares methods for spectral analyses. 1. Relation to other quantitative calibration methods and the extraction of qualitative information. Anal Chem 60:1193–1204
Kuzmanovski I, Novic M (2008) Counter-propagation neural network in Matlab. Chem Intell Lab Sys 90:84–91
Harris RLN (1976) Potential wool growth inhibitors. Improved syntheses of mimosine and related 4(1H)-pyridones. Aust J Chem 29:1329–1334
Sabet R, Behjati M, Vahabpour R, Memarnejadian A, Rostami M, Fassihi A, Aghasadeghi MR, Saghaie L, Miri R (in press) Int J Cardiol. doi:10.1016/j.ijcard.2011.11.067
Kirilmis C, Ahmedzade M, Servi S, Koca M, Kizirgil A, Kazaz C (2008) Synthesis and antimicrobial activity of some novel derivatives of benzofuran: part 2. The synthesis and antimicrobial activity of some novel 1-(1-benzofuran-2-yl)-2-mesitylethanone derivatives. Eur J Med Chem 43:300–308
de Souza SM, Delle Monache F, Smania A Jr (2005) Antibacterial activity of coumarins. Z Naturforsch 60:693–700
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sabet, R., Fassihi, A., Hemmateenejad, B. et al. Computer-aided design of novel antibacterial 3-hydroxypyridine-4-ones: application of QSAR methods based on the MOLMAP approach. J Comput Aided Mol Des 26, 349–361 (2012). https://doi.org/10.1007/s10822-012-9561-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-012-9561-2