Abstract
Purpose/study question
Does piercing oocyte membranes during ICSI allow the influx of surrounding zwitterionic buffer into human oocytes and result in altered developmental competence?
Methods
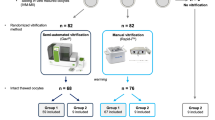
Human oocytes directed to IRB-approved research were used to determine the unrestricted influx of surrounding buffer into the oocyte after piercing of membranes via confocal fluorescence microscopy (n = 80 human MII oocytes) and the influence of the select buffer influx of HEPES, MOPS, and bicarbonate buffer on the oocyte transcriptome using ultra-low input RNA sequencing (n = 40 human MII oocytes).
Results
Piercing membranes of human MII oocytes during sham-ICSI resulted in the unrestricted influx of surrounding culture buffer into the oocyte that was beyond technician control. Transcriptome analysis revealed statistically significant decreased cytoskeletal transcripts in the pierced buffer cohorts, higher levels of embryo competency transcripts (IGF2 and G6PD) in the bicarbonate buffer cohort, higher levels of stress-induced transcriptional repressor transcripts (MAF1) in the HEPES and MOPS cohorts, and decreased levels of numerous chromosomal maintenance transcripts (SMC3) in the HEPES buffer cohort. The HEPES buffer cohort also revealed higher levels of transcripts suggesting increased oxidative (GPX1) and lysosomal stress (LAMP1).
Conclusion
The influence of zwitterionic buffer on intrinsic cellular mechanisms provides numerous concerns for their use in IVF clinical applications. The primary concern is the ICSI procedure, in which the surrounding buffer is allowed influx into the oocytes after membrane piercing. Selecting a physiological bicarbonate buffer may reduce imposed stress on oocytes, resulting in improved embryo development and clinical results because intracellular MOPS, and especially HEPES, may negatively impact intrinsic biological mechanisms, as revealed by transcriptome changes. These findings further support the utilization of bicarbonate buffer as the oocyte-holding medium during ICSI.





Similar content being viewed by others
Data availability
All data is available on request. Any data underlying this article not contained within this publication will be shared on reasonable request to the author.
References
Ng K, Mingels R, Morgan H, Macklon N, Cheong Y. In vivo oxygen, temperature and pH dynamics in the female reproductive tract and their importance in human conception: a systematic review. Hum Reprod Update. 2017;24(1):15–34. https://doi.org/10.1093/humupd/dmx028.
Will M, Clark N, Swain J. Biological pH buffers in IVF: help or hindrance to success. J Assist Reprod Genet. 2011;28(8):711–24. https://doi.org/10.1007/s10815-011-9582-0.
Swain J, Carrell D, Cobo A, Meseguer M, Rubio C, Smith G. Optimizing the culture environment and embryo manipulation to help maintain embryo developmental potential. Fertil Steril. 2016;105(3):571–87. https://doi.org/10.1016/j.fertnstert.2016.01.035.
Swain JE, Wilding M. Practical ph for the IVF laboratory. J Reprod Stem Cell Biotechnol. 2012;3(2):62–76.
Dale B, Menezo Y, Cohen J, DiMatteo L, Wilding M. Intracellular pH regulation in the human oocyte. Hum Reprod. 1998;13:964–70.
FitzHarris G, Baltz JM. Regulation of intracellular ph during oocyte growth and maturation in mammals. Reproduction. 2009;138(4):619–27.
FitzHarris G, Siyanov V, Baltz JM. Granulosa cells regulate oocyte intracellular pH against acidosis in preantral follicles by multiple mechanisms. Development. 2007;134:4283–95.
Mendola R, Walmsley R, Schimmel T, Sagerer E, Mullen C, Russell H, Angle M, Garrisi J. Improved embryo development and clinical outcome using bicarbonate buffer as the oocyte holding medium during Intracytoplasmic sperm injection (ICSI) compared to MOPS buffer. P-157. Human Reprod. 2023;38(Supplement_1). https://doi.org/10.1093/humrep/dead093.520.
Morgia F, Torti M, Montigiani M, et al. Use of a medium buffered with N-hydroxyethylpiperazine-N-ethanesulfonate (HEPES) in intracytoplasmic sperm injection procedures is detrimental to the outcome of in vitro fertlization. Fertil Steril. 2006;85(5):1415–9. https://doi.org/10.1016/j.fertnstert.2005.10.050.
Hanrahan J, Tabcharani J. Inhibition of an outwardly rectifying anion channel by HEPES and related buffers. J Membr Biol. 1990;116(1):65–77. https://doi.org/10.1007/bf01871673.
Li M, Farley RA, Lester HA. Voltage-dependent transient currents of human and rat 5-HT transporters (SERT) are blocked by HEPES and ion channel ligands. FEBS Lett. 2002;513(2–3):247–52. https://doi.org/10.1016/s0014-5793(02)02322-0.
Nikishin DA, Alyoshina NM, Shmukler YB. Synthesis and membrane transport of serotonin in the developing ovarian follicle of mouse. Dokl Biochem Biophys. 2018;478(1):4–7. https://doi.org/10.1134/s1607672918010027.
Koerner MM, Palacio LA, Wright JW, Schweitzer KS, Ray BD, Petrache HI. Electrodynamics of lipid membrane interactions in the presence of zwitterionic buffers. Biophys J. 2011;101(2):362–9. https://doi.org/10.1016/j.bpj.2011.05.062.
Van Campenhout R, Gomes AR, De Groof TWM, Muyldermans S, Devoogdt N, Vinken M. Mechanisms underlying connexin hemichannel activation in disease. Int J Mol Sci. 2021;22(7):3503. https://doi.org/10.3390/ijms22073503.
Bevans CG, Harris AL. Regulation of connexin channels by ph. J Biol Chem. 1999;274(6):3711–9. https://doi.org/10.1074/jbc.274.6.3711.
Ferreira CM, Pinto IS, Soares EV, Soares HM. (un)suitability of the use of ph buffers in biological, biochemical and environmental studies and their interaction with metal ions – a review. RSC Adv. 2015;5(39):30989–1003. https://doi.org/10.1039/c4ra15453c.
Michl J, Park K, Swietach P. Evidence-based guidelines for controlling pH in mammalian live-cell culture systems. Commun Biol. 2019;2(1). https://doi.org/10.1038/s42003-019-0393-7.
Yang X, Chasteen ND. Ferroxidase activity of ferritin: effects of ph, buffer and fe(ii) and fe(iii) concentrations on fe(ii) autoxidation and ferroxidation. Biochem J. 1999;338(3):615–8. https://doi.org/10.1042/bj3380615.
Zhao M-H, Liang S, Kim S-H, Cui X-S, Kim N-H. Fe(III) is essential for porcine embryonic development via mitochondrial function maintenance. PLoS One. 2015;10(7). https://doi.org/10.1371/journal.pone.0130791.
Dong F, Zhang X, Culver B, Chew HG Jr, Kelley RO, Ren J. Dietary iron deficiency induces ventricular dilation, mitochondrial ultrastructural aberrations and cytochrome c release: involvement of nitric oxide synthase and protein tyrosine nitration. Clin Sci (Lond). 2005;109(3):277–86.
Walter PB, Knutson MD, Paler-Martinez A, Lee S, Xu Y, Viteri FE, et al. Iron deficiency and iron excess damage mitochondria and mitochondrial DNA in rats. Proc Natl Acad Sci USA. 2002;99(4):2264–9.
Stellwagen NC, Bossi A, Gelfi C, Righetti PG. DNA and buffers: are there any noninteracting, neutral ph buffers? Anal Biochem. 2000;287(1):167–75. https://doi.org/10.1006/abio.2000.4848.
Grady JK, Chasteen ND, Harris DC. Radicals from “good's” buffers.Analytical Biochemistry. 1988;173(1):111–115. https://doi.org/10.1016/0003-2697(88)90167-4.Handy DE, Loscalzo J. The role of glutathione peroxidase-1 in health and disease. Free Rad Biol Med. 2022;188:146–161. https://doi.org/10.1016/j.freeradbiomed.2022.06.004.
Zhao G, Chasteen ND. Oxidation of good’s buffers by hydrogen peroxide. Anal Biochem. 2006;349(2):262–7. https://doi.org/10.1016/j.ab.2005.10.005.
Sies H. Role of metabolic H2O2 generation. J Biol Chem. 2014;289(13):8735–41. https://doi.org/10.1074/jbc.r113.544635.
Van Haaren PM, VanBavel E, Vink H, Spaan JA. Charge modification of the endothelial surface layer modulates the permeability barrier of isolated rat mesenteric small arteries. Am J Physiol-Heart Circ Physiol. 2005;289(6). https://doi.org/10.1152/ajpheart.00587.2005.
Cowan AI, Martin RL. Ionic basis of the membrane potential responses of rat dorsal vagal motoneurones to HEPES buffer. Brain Res. 1996;717(1–2):69–75. https://doi.org/10.1016/0006-8993(96)00052-2.
Crennell SJ, et al. The structure of Rhodothermus marinus Cel12A, a highly thermostable family 12 endoglucanase at 1.8 Å resolution. J Mol Biol. 2002;320:883–97.
Zigler JS, Lepe-Zuniga JL, Vistica B, Gery I. Analysis of the cytotoxic effects of light-exposed Hepes-containing culture medium. In Vitro Cell Dev Biol. 1985;21(5):282–7. https://doi.org/10.1007/bf02620943.
Spierenburg GT, Oerlemans FTJJ, Van Laarhoven JPRM, De Bruyn CHMM. Phototoxicity of N-2-hydroxyethylpiperazfine-N’-2-ethanesulfonic acid-buffered culture media for human leukemic cell lines. Cancer Res. 1984;44:2253.
Tol MJ, van der Lienden MJC, Gabriel TL, Hagen JJ, Scheij S, Veenendaal T, Klumperman J, Donker-Koopman WE, Verhoeven AJ, Overkleeft H, Aerts JM, Argmann CA, van Eijk M. Hepes activates a MIT/TFE-dependent lysosomal-autophagic gene network in cultured cells: a call for caution. Autophagy. 2018;14(3):437–49. https://doi.org/10.1080/15548627.2017.1419118.
Azenta Life Sciences: GENEWIZ | RNA sequencing. https://www.genewiz.com/en/Public/Services/Next-Generation-Sequencing/RNA-Seq. Accessed March 13, 2022.
Kimmel CB, Warga RM, Schilling TF. Origin and organization of the zebrafish fate map. Development. 1990;108(4):581–94. https://doi.org/10.1242/dev.108.4.581.
Elder K, Dale B. In vitro fertilization. 4th ed. Cambridge: Cambridge University Press; 2020.
FujiFilm Irvine Scientific: Vit Kit-Thawing Protocol. Irvinesci.com. https://www.irvinesci.com/vit-kit-thaw.html. Accessed 4 Sept 2022.
Khanna A, Pradhan A, Curran S. Emerging roles for Maf1 beyond the regulation of RNA polymerase III activity. J Mol Biol. 2015;427(16):2577–85. https://doi.org/10.1016/j.jmb.2015.06.022.
Boisnard Stéphanie, Lagniel G, Garmendia-Torres C, et al. H 2 O 2 activates the nuclear localization of MSN2 and MAF1 through thioredoxins in saccharomyces cerevisiae. Eukaryotic Cell. 2009;8(9):1429–1438. https://doi.org/10.1128/ec.00106-09.
Handy DE, Loscalzo J. The role of glutathione peroxidase-1 in health and disease. Free Radical Biol Med. 2022;188:146–61. https://doi.org/10.1016/j.freeradbiomed.2022.06.004.
Kleijkers SH, Eijssen LM, Coonen E, Derhaag JG, Mantikou E, Jonker MJ, Mastenbroek S, Repping S, Evers JL, Dumoulin JC, et al. Differences in gene expression profiles between human preimplantation embryos cultured in two different IVF culture media. Hum Reprod. 2015;30:2303–11.
Monzo C, Haouzi D, Roman K, Assou S, Dechaud H, Hamamah S. Slow freezing and vitrification differentially modify the gene expression profile of human metaphase II oocytes. Hum Reprod. 2012;27(7):2160–8.
Tan M, van Tol H, Mokry M, Stout T, Roelen B. Microinjection induces changes in the transcriptome of bovine oocytes. Sci Rep. 2020;10(1). https://doi.org/10.1038/s41598-020-67603-4.
Quail DF, Bowman RL, Akkari L, et al. The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science. 2016;352(6288). https://doi.org/10.1126/science.aad3018.
Asami M, Lam B, Ma M, et al. Human embryonic genome activation initiates at the one-cell stage. Cell Stem Cell. 2022;29(2):209-216.e4. https://doi.org/10.1016/j.stem.2021.11.012.
Cheng J-M, et al. Elevated intracellular pH appears in aged oocytes and causes oocyte aneuploidy associated with the loss of cohesion in mice. Cell Cycle. 2016;15(18):2454–63.
Zhao R, Najmi M, Aluri S, Spray DC, Goldman ID. Concentrative transport of antifolates mediated by the Proton-coupled folate transporter (SLC46A1); augmentation by a HEPES buffer. Mol Pharmacol. 2018;93(3):208–15. https://doi.org/10.1124/mol.117.110445.
Liu P, Sun J, Peng W, et al. Zwitterionic betaines over Hepes as the new generation biocompatible ph buffers for cell culture. Bioact Mater. 2023;24:376–86. https://doi.org/10.1016/j.bioactmat.2022.12.028.
Cheng J-M, Liu Y-X. Age-related loss of cohesion: causes and effects. Int J Mol Sci. 2017;18(7):1578. https://doi.org/10.3390/ijms18071578.
Stanton RC. Glucose-6-phosphate dehydrogenase, NADPH, and cell survival. IUBMB Life. 2012;64(5):362–9. https://doi.org/10.1002/iub.1017.
Tsujii H, Lee JH, Hossain MS, Tareq KM, Hamano K, Sawada T. The beneficial effect of fructose and glucose on in vitro maturation and the fertilization of porcine oocytes. Reprod Med Biol. 2008;8(1):19–24. https://doi.org/10.1007/s12522-008-0003-8.
Palasz A, Breña PB, Fuente JDL, Gutiérrez-Adán A. The effect of different zwitterionic buffers and PBS used for out-of-incubator procedures during standard in vitro embryo production on development, morphology and gene expression of bovine embryos. Theriogenology. 2008;70(9):1461–70. https://doi.org/10.1016/j.theriogenology.2008.06.092.
Hsu J, Arand J, Chaikovsky A et al. E2F4 regulates transcriptional activation in mouse embryonic stem cells independently of the Rb family. Nat Commun. 2019;10(1). https://doi.org/10.1038/s41467-019-10901-x.
Swain J. Is there an optimal pH for culture media used in clinical IVF? Hum Reprod Update. 2012;18(3):333–9. https://doi.org/10.1093/humupd/dmr053.
Acknowledgements
Completion of this project would not be possible without the contributions of many other distinguished collaborators such as Tim Schimmel, the IRMS Embryology Team, the IRMS Physicians, fluorescent staining and oocyte fixation discussion with Dr. John Henson and Dr. Calvin Simerly, and RNA-sequencing with the team at Azenta Life Sciences (Genewiz) Inc.
Author information
Authors and Affiliations
Contributions
R.J.M was the principal investigator responsible for the design of the study, conducting experiments, data analysis, writing, and editing. K.S. and L.B. assisted with the confocal fluorescence microscopy and publication editing. R.H.W. assisted with the collection of IRB research material for the study. H.R. and M.A. assisted with IRB approval from EVMS, writing and editing. G.J.G. assisted with the collection of IRB research material for the study, provided research oversight, and assisted with writing and editing. K.S. and L.B. are supported by grants from the NIH: R01HD091331-A1 and F30HD107976-A1, respectively. All listed authors approve of this research and this publication.
Corresponding author
Ethics declarations
Ethical approval
The use of human oocytes directed to IRB research was approved by the Eastern Virginia Medical School (EVMS) IRB (protocol # 22–01-NH-0023) and by the IRB-approved research at the Institute for Reproductive Medicine and Science (IRMS) at Saint Barnabas (WCG ASPIRE® Protocol #20193402) certifying that the study was performed following the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. All IRB research consents used in this study include patient consent to participate and publish.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mendola, R.J., Biswas, L., Schindler, K. et al. Influx of zwitterionic buffer after intracytoplasmic sperm injection (ICSI) membrane piercing alters the transcriptome of human oocytes. J Assist Reprod Genet (2024). https://doi.org/10.1007/s10815-024-03064-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s10815-024-03064-2