Abstract
Purpose
To investigate the genetic etiology of patients with female infertility.
Methods
Whole Exome Sequencing was performed on genomic DNA extracted from the patient’s blood. Exome data were filtered for damaging rare biallelic variants in genes with possible roles in reproduction. Sanger sequencing was used to validate the selected variants and segregate them in family members.
Results
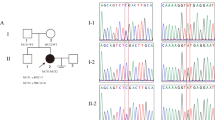
A novel homozygous likely pathogenic variant, c.626G>A, p.Trp209*, was identified in the TERB1 gene of the patient. Additionally, we report a second homozygous pathogenic TERB1 variant, c.1703C>G, p.Ser568*, in an infertile woman whose azoospermic brother was previously described to be homozygous for her variant.
Conclusions
Here, we report for the first time two homozygous likely pathogenic and pathogenic TERB1 variants, c.626G>A, p.Trp209* and c.1703C>G, p.Ser568*, respectively, in two unrelated women with primary infertility. TERB1 is known to play an essential role in homologous chromosome movement, synapsis, and recombination during the meiotic prophase I and has an established role in male infertility in humans. Our data add TERB1 to the shortlist of Meiosis I genes associated with human infertility in both sexes.

Similar content being viewed by others
References
Tamrakar SR, Bastakoti R. Determinants of Infertility in Couples. J Nepal Health Res Counc. 2019;17(1):85–89. Published 2019 Apr 28. https://doi.org/10.33314/jnhrc.1827
Sang Q, Ray PF, Wang L. Understanding the genetics of human infertility. Science (New York, N.Y.). 2023;380(6641):158–163. https://doi-org.proxy3.library.mcgill.ca/https://doi.org/10.1126/science.adf7760
DavariTanha F, Mohseni M, Ghajarzadeh M. Sexual function in women with primary and secondary infertility in comparison with controls. Int J Impot Res. 2014;26(4):132–4. https://doi.org/10.1038/ijir.2013.51.
Carson SA, Kallen AN. Diagnosis and Management of Infertility: A review. JAMA. 2021;326(1):65–76. https://doi.org/10.1001/jama.2021.4788.
Leaver RB. Male infertility: an overview of causes and treatment options. Br J Nurs. 2016;25(18):S35–40. https://doi.org/10.12968/bjon.2016.25.18.S35.
Krausz C, Riera-Escamilla A. Genetics of male infertility. Nat Rev Urol. 2018;15(6):369–384. https://doi-org.proxy3.library.mcgill.ca/https://doi.org/10.1038/s41585-018-0003-3
National Health Service. LH and FSH (Gonadotrophins). NHS Gloucestershire Hospitals. 2023. Retrieved December 3, 2023 from https://www.gloshospitals.nhs.uk/our-services/services-we-offer/pathology/tests-and-investigations/lh-and-fsh-gonadotrophins/.
Dayal M, Sagar S, Chaurasia A, Singh U. Anti-mullerian hormone: A new marker of ovarian function. J Obstet Gynaecol India. 2014;64(2):130–3. https://doi.org/10.1007/s13224-013-0482-3.
Salas-Huetos A, Tüttelmann F, Wyrwoll MJ, et al. Disruption of human meiotic telomere complex genes TERB1, TERB2 and MAJIN in men with non-obstructive azoospermia [published correction appears in Hum Genet. 2020 Dec 30;:]. Hum Genet. 2021;140(1):217–227. https://doi.org/10.1007/s00439-020-02236-1.
Li H, Durbin R. Fast and accurate short read alignment with burrows-wheeler transform. Bioinformatics. 2009;25:1754–60.
Picard Toolkit. Broad Institute, GitHub Repository. Broad Institute. 2019. https://broadinstitute.github.io/picard/.
Nguyen NMP, Ge ZJ, Reddy R, Fahiminiya S, Sauthier P, Bagga R, Sahin FI, Mahadevan S, Osmond M, Breguet M, Rahimi K, Lapensee L, Hovanes K, Srinivasan R, Van den Veyver IB, Sahoo T, Ao A, Majewski J, Taketo T, Slim R. Causative mutations and mechanism of androgenetic hydatidiform moles. Am J Hum Genet. 2018;103(5):740–751. https://doi-org.proxy3.library.mcgill.ca/https://doi.org/10.1016/j.ajhg.2018.10.007.
Karczewski KJ, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581(7809):434–43.
Rentzsch P, Witten D, Cooper GM, Shendure J, Kircher M. CADD: Predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. 2019;47(D1):D886–94. https://doi.org/10.1093/nar/gky1016.
Kopanos C, Tsiolkas V, Kouris A, et al. VarSome: The human genomic variant search engine. Bioinformatics. 2019;35(11):1978–80. https://doi.org/10.1093/bioinformatics/bty897.
Tavtigian SV, Harrison SM, Boucher KM, Biesecker LG. Fitting a naturally scaled point system to the ACMG/AMP variant classification guidelines. Hum Mutat. 2020;41(10):1734–7. https://doi.org/10.1002/humu.24088.
Untergasser A, Nijveen H, Rao X, Bisseling T, Geurts R, Jack AM. Leunissen: Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res. 2007;35:W71-W74. https://doi.org/10.1093/nar/gkm306
Murdoch S, Djuric U, Mazhar B, Seoud M, Khan R, Kuick R, Bagga R, Kircheisen R, Ao A, Ratti B, Hanash S, Rouleau GA, Slim R. Mutations in NALP7 cause recurrent hydatidiform moles and reproductive wastage in humans. Nat Genet. 2006;38(3):300–2. https://doi.org/10.1038/ng1740.
Hu Z, Yau C, Ahmed AA (2016) masonmd: Making sense of nonsense-mediated decay. https://github.com/zhiyhu/masonmd?tab=readme-ov-file. doi: 10.5281/zenodo.546698.
Schwarz JM, Cooper DN, Schuelke M, Seelow D. MutationTaster2: Mutation prediction for the deep-sequencing age. Nat Methods. 2014;11(4):361–2. https://doi.org/10.1038/nmeth.2890.
Zhang J, Tu Z, Watanabe Y, Shibuya H. Distinct TERB1 domains regulate different protein interactions in meiotic telomere movement. Cell Rep. 2017;21(7):1715–26. https://doi.org/10.1016/j.celrep.2017.10.061.
Kherraf ZE, Cazin C, Bouker A, et al. Whole-exome sequencing improves the diagnosis and care of men with non-obstructive azoospermia. Am J Hum Genet. 2022;109(3):508–17. https://doi.org/10.1016/j.ajhg.2022.01.011.
Krausz C, Riera-Escamilla A, Moreno-Mendoza D, et al. Genetic dissection of spermatogenic arrest through exome analysis: clinical implications for the management of azoospermic men. Genet Med. 2020;22(12):1956–66. https://doi.org/10.1038/s41436-020-0907-1.
Alhathal N, Maddirevula S, Coskun S, et al. A genomics approach to male infertility. Genet Med. 2020;22(12):1967–75. https://doi.org/10.1038/s41436-020-0916-0.
Shibuya H, Ishiguro K, Watanabe Y. The TRF1-binding protein TERB1 promotes chromosome movement and telomere rigidity in meiosis. Nat Cell Biol. 2014;16(2):145–56. https://doi.org/10.1038/ncb2896.
Wang Y, Chen Y, Chen J, et al. The meiotic TERB1-TERB2-MAJIN complex tethers telomeres to the nuclear envelope. Nat Commun. 2019;10(1):564. Published 2019 Feb 4. https://doi.org/10.1038/s41467-019-08437-1
Dunce JM, Milburn AE, Gurusaran M, et al. Structural basis of meiotic telomere attachment to the nuclear envelope by MAJIN-TERB2-TERB1. Nat Commun. 2018;9(1):5355. Published 2018 Dec 17. https://doi.org/10.1038/s41467-018-07794-7.
Spindler MC, Redolfi J, Helmprobst F, Kollmannsberger P, Stigloher C, Benavente R. Electron tomography of mouse LINC complexes at meiotic telomere attachment sites with and without microtubules. Commun Biol. 2019;2:376. Published 2019 Oct 14. https://doi.org/10.1038/s42003-019-0621-1
Harmak H, Charoute H, Redouane S, Filali OA, Barakat A, Rouba H. Computational analysis of the potential impact of MTC complex missenses SNPs associated with male infertility. Biomed Res Int. 2022;2022:1664825. https://doi.org/10.1155/2022/1664825.
Ding X, Xu R, Yu J, Xu T, Zhuang Y, Han M. SUN1 is required for telomere attachment to nuclear envelope and gametogenesis in mice. Dev Cell. 2007;12(6):863–72. https://doi.org/10.1016/j.devcel.2007.03.018.
Horn HF, Kim DI, Wright GD, et al. A mammalian KASH domain protein coupling meiotic chromosomes to the cytoskeleton. J Cell Biol. 2013;202(7):1023–39. https://doi.org/10.1083/jcb.201304004.
Meng Q, Shao B, Zhao D, et al. Loss of SUN1 function in spermatocytes disrupts the attachment of telomeres to the nuclear envelope and contributes to non-obstructive azoospermia in humans. Hum Genet. 2023;142(4):531–41. https://doi.org/10.1007/s00439-022-02515-z.
Wu H, Zhang X, Hua R, et al. Homozygous missense mutation in CCDC155 disrupts the transmembrane distribution of CCDC155 and SUN1, resulting in non-obstructive azoospermia and premature ovarian insufficiency in humans. Hum Genet. 2022;141(11):1795–809. https://doi.org/10.1007/s00439-022-02459-4.
Zhang Q, Tao C, Gao S, et al. Homozygous variant in KASH5 causes premature ovarian insufficiency by disordered meiotic homologous pairing. J Clin Endocrinol Metab. 2022;107(9):2589–97. https://doi.org/10.1210/clinem/dgac368.
Hou X, Zeb A, Dil S, et al. A homozygous KASH5 frameshift mutation causes diminished ovarian reserve, recurrent miscarriage, and non-obstructive azoospermia in humans. Front Endocrinol (Lausanne). 2023;14:1128362. Published 2023 Feb 14. https://doi.org/10.3389/fendo.2023.1128362.
Hua R, Liu M. Sexual dimorphism in mouse meiosis. Front Cell Dev Biol. 2021;9:670599. Published 2021 May 10. https://doi.org/10.3389/fcell.2021.670599.
Handel MA, Eppig JJ. Sexual dimorphism in the regulation of mammalian meiosis. Curr Top Dev Biol. 1998;37:333–58. https://doi.org/10.1016/s0070-2153(08)60179-9.
Hunt PA, Hassold TJ. Sex matters in meiosis. Science. 2002;296(5576):2181–3. https://doi.org/10.1126/science.1071907.
Acknowledgements
We thank the patients and their relatives for participating in this study, Christina Burhöi for her technical support, Dr. Sophie A. Koser for contacting the study participants, and Mohamed Ramadan for providing his consultation. We acknowledge the use of the Centre d’expertise et de services Génome Québec. This work was supported by the Canadian Institute of Health Research (PJT—180509, OGB – 177939, and PJT—155998). ZY was supported by Mitacs Accelerate (Ref. IT31962). ML was supported by the Research Institute of the McGill University Health Centre Desjardins Studentship, McGill University Faculty of Medicine Internal Studentship, and Travel Funding Support from Réseau Québécois en Reproduction and the Department of Human Genetics of McGill University. CF and FT were supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) sponsored Clinical Research Unit ‘Male Germ Cells’ (CRU326, project number 329621271).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yalcin, Z., Liang, M., Abdelrazek, I. et al. A report of two homozygous TERB1 protein-truncating variants in two unrelated women with primary infertility. J Assist Reprod Genet 41, 751–756 (2024). https://doi.org/10.1007/s10815-024-03031-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-024-03031-x