Abstract
Purpose
To evaluate the obstetric and perinatal outcomes of three routine endometrial preparation protocols in women with PCOS who underwent frozen embryo transfer (FET).
Methods
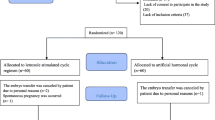
This was a retrospective study in women with PCOS who underwent FET in an academic reproductive medical center. A total of 2710 cycles were enrolled and classified into three groups according to different endometrial preparation protocols; human menopausal gonadotropin (HMG), letrozole + HMG, or hormone replacement therapy (HRT).
Results
The stimulation groups had reduced risks of hypertensive disorders of pregnancy (HDP), large for gestational age (LGA) infants, and cesarean delivery than the HRT group. After adjustment for different confounder combinations in the two models, the frequencies of LGA and HDP in the letrozole + HMG group and the HMG group were still significantly lower than those in the HRT group. The letrozole + HMG group exhibited a reduced risk of LGA than HMG group after adjustment of confounders. A trend toward risk reductions in HDP and LGA was observe in turns of HRT, HMG, and letrozole + HMG groups, and the trends were statistically significant (Ptrend = 0.031 and 0.001).
Conclusion
In patients with PCOS, ovarian stimulation protocols for endometrial preparation are associated with reduced risks of HDP and LGA compared to HRT cycles. The use of letrozole could further reduce risk of LGA compared to HMG only protocol. We propose that ovarian stimulation protocols can be used widely for endometrial preparation in FET cycles in women with PCOS, especially with the use of letrozole.

Similar content being viewed by others
Abbreviations
- PCOS:
-
Polycystic ovary syndrome
- FET:
-
Frozen embryo transfer
- HMG:
-
Human menopausal gonadotropin
- HRT:
-
Hormone replacement therapy
- HDP:
-
Hypertensive disorders of pregnancy
- LGA:
-
Large for gestational age
- IVF:
-
In vitro fertilization
- ET:
-
Embryo transfer
- OHSS:
-
Ovarian hyperstimulation syndrome
- OS:
-
Ovarian stimulation
- LH:
-
Luteinizing hormone
- GDP:
-
Gestational diabetes mellitus
- SGA:
-
small for gestational age
- PPH:
-
Postpartum hemorrhage
- FSH:
-
Follicle-stimulating hormone
- AFC:
-
Antral follicle counta
- OR:
-
Adjusted odds ratio
- CI:
-
Confidence interval
- CL:
-
Corpus luteum
- cfPWV:
-
Carotid-femoral pulse wave velocity
- cfPWTT:
-
Carotid-femoral pulse wave transit time
- ICSI:
-
Intracytoplasmic sperm injection
- PGT:
-
Preimplantation genetic testing
References
Adams J, Polson DW, Franks S. Prevalence of polycystic ovaries in women with anovulation and idiopathic hirsutism. Br Med J (Clin Res Ed). 1986;293(6543):355–9.
Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS, Lizneva D, Natterson-Horowtiz B, Teede HJ, Yildiz BO. Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2:16057.
Delvigne A, Rozenberg S. Epidemiology and prevention of ovarian hyperstimulation syndrome (OHSS): a review. Hum Reprod Update. 2002;8(6):559–77.
Tang T, Glanville J, Hayden CJ, White D, Barth JH, Balen AH. Combined lifestyle modification and metformin in obese patients with polycystic ovary syndrome. A randomized, placebo-controlled, double-blind multicentre study. Hum Reprod. 2006;21(1):80–9.
Groenewoud ER, Cantineau AE, Kollen BJ, Macklon NS, Cohlen BJ. What is the optimal means of preparing the endometrium in frozen-thawed embryo transfer cycles? A systematic review and meta-analysis. Hum Reprod Update. 2013;19(5):458–70.
Casper RF, Yanushpolsky EH. Optimal endometrial preparation for frozen embryo transfer cycles: window of implantation and progesterone support. Fertil Steril. 2016;105(4):867–72.
Groenewoud ER, Cohlen BJ, Al-Oraiby A, Brinkhuis EA, Broekmans FJ, de Bruin JP, van den Dool G, Fleisher K, Friederich J, Goddijn M, et al. A randomized controlled, non-inferiority trial of modified natural versus artificial cycle for cryo-thawed embryo transfer. Hum Reprod. 2016;31(7):1483–92.
Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14(5):270–84.
Zhang Y, Wu L, Li TC, Wang CC, Zhang T, Chung JPW. Systematic review update and meta-analysis of randomized and non-randomized controlled trials of ovarian stimulation versus artificial cycle for endometrial preparation prior to frozen embryo transfer in women with polycystic ovary syndrome. Reprod Biol Endocrinol. 2022;20(1):62.
Roos N, Kieler H, Sahlin L, Ekman-Ordeberg G, Falconer H, Stephansson O. Risk of adverse pregnancy outcomes in women with polycystic ovary syndrome: population based cohort study. Bmj. 2011;343:d6309.
Pan ML, Chen LR, Tsao HM, Chen KH. Relationship between polycystic ovarian syndrome and subsequent gestational diabetes mellitus: a nationwide population-based study. PLoS One. 2015;10(10):e0140544.
Palomba S, de Wilde MA, Falbo A, Koster MP, La Sala GB, Fauser BC. Pregnancy complications in women with polycystic ovary syndrome. Hum Reprod Update. 2015;21(5):575–92.
Jing S, Li XF, Zhang S, Gong F, Lu G, Lin G. Increased pregnancy complications following frozen-thawed embryo transfer during an artificial cycle. J Assist Reprod Genet. 2019;36(5):925–33.
Wang B, Zhang J, Zhu Q, Yang X, Wang Y. Effects of different cycle regimens for frozen embryo transfer on perinatal outcomes of singletons. Hum Reprod. 2020;35(7):1612–22.
Pan Y, Li B, Wang Z, Wang Y, Gong X, Zhou W, Shi Y. Hormone replacement versus natural cycle protocols of endometrial preparation for frozen embryo transfer. Front Endocrinol (Lausanne). 2020;11:546532.
Saito K, Kuwahara A, Ishikawa T, Morisaki N, Miyado M, Miyado K, Fukami M, Miyasaka N, Ishihara O, Irahara M, et al. Endometrial preparation methods for frozen-thawed embryo transfer are associated with altered risks of hypertensive disorders of pregnancy, placenta accreta, and gestational diabetes mellitus. Hum Reprod. 2019;34(8):1567–75.
Conrad KP, Baker VL. Corpus luteal contribution to maternal pregnancy physiology and outcomes in assisted reproductive technologies. Am J Physiol Regul Integr Comp Physiol. 2013;304(2):R69–72.
Singh B, Reschke L, Segars J, Baker VL. Frozen-thawed embryo transfer: the potential importance of the corpus luteum in preventing obstetrical complications. Fertil Steril. 2020;113(2):252–7.
Zhang J, Wei M, Bian X, Wu L, Zhang S, Mao X, Wang B. Letrozole-induced frozen embryo transfer cycles are associated with a lower risk of hypertensive disorders of pregnancy among women with polycystic ovary syndrome. Am J Obstet Gynecol. 2021;225(1):59.e51–9.
Man Y, Bian Y, Zhao S, Zhao R, Xu X, Wei D, Li L, Chen ZJ, Zhao H. The effect of different endometrial preparations on women with polycystic ovary syndrome undergoing initial frozen embryo transfer: a historical cohort analysis. Acta Obstet Gynecol Scand. 2021;100(6):1116–23.
De Geyter C, Calhaz-Jorge C, Kupka MS, Wyns C, Mocanu E, Motrenko T, Scaravelli G, Smeenk J, Vidakovic S, Goossens V. ART in Europe, 2014: Results generated from European registries by ESHRE: The European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). Hum Reprod. 2018;33(9):1586–601.
Revised. Consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004. 2003;19(1):41–7.
Dai L, Deng C, Li Y, Zhu J, Mu Y, Deng Y, Mao M, Wang Y, Li Q, Ma S, et al. Birth weight reference percentiles for Chinese. PLoS One. 2014;9(8):e104779.
Hu KL, Zhang D, Li R. Endometrium preparation and perinatal outcomes in women undergoing single-blastocyst transfer in frozen cycles. Fertil Steril. 2021;115(6):1487–94.
Katulski K, Czyzyk A, Podfigurna-Stopa A, Genazzani AR, Meczekalski B. Pregnancy complications in polycystic ovary syndrome patients. Gynecol Endocrinol. 2015;31(2):87–91.
Falbo A, Rocca M, Russo T, D'Ettore A, Tolino A, Zullo F, Orio F, Palomba S. Changes in androgens and insulin sensitivity indexes throughout pregnancy in women with polycystic ovary syndrome (PCOS): relationships with adverse outcomes. J Ovarian Res. 2010;3:23.
Kjerulff LE, Sanchez-Ramos L, Duffy D. Pregnancy outcomes in women with polycystic ovary syndrome: a metaanalysis. Am J Obstet Gynecol. 2011;204(6):558.e551–6.
Wang Z, Liu H, Song H, Li X, Jiang J, Sheng Y, Shi Y. Increased risk of pre-eclampsia after frozen-thawed embryo transfer in programming cycles. Front Med (Lausanne). 2020;7:104.
MacLaughlan SD, Palomino WA, Mo B, Lewis TD, Lininger RA, Lessey BA. Endometrial expression of Cyr61: a marker of estrogenic activity in normal and abnormal endometrium. Obstet Gynecol. 2007;110(1):146–54.
Nardo LG, Bartoloni G, Di Mercurio S, Nardo F. Expression of alpha(v)beta3 and alpha4beta1 integrins throughout the putative window of implantation in a cohort of healthy fertile women. Acta Obstet Gynecol Scand. 2002;81(8):753–8.
Tei C, Maruyama T, Kuji N, Miyazaki T, Mikami M, Yoshimura Y. Reduced expression of alphavbeta3 integrin in the endometrium of unexplained infertility patients with recurrent IVF-ET failures: improvement by danazol treatment. J Assist Reprod Genet. 2003;20(1):13–20.
Conrad KP. Evidence for corpus luteal and endometrial origins of adverse pregnancy outcomes in women conceiving with or without assisted reproduction. Obstet Gynecol Clin North Am. 2020;47(1):163–81.
von Versen-Höynck F, Schaub AM, Chi YY, Chiu KH, Liu J, Lingis M, Stan Williams R, Rhoton-Vlasak A, Nichols WW, Fleischmann RR, et al. Increased preeclampsia risk and reduced aortic compliance with in vitro fertilization cycles in the absence of a corpus luteum. Hypertension. 2019;73(3):640–9.
Conrad KP. Maternal vasodilation in pregnancy: the emerging role of relaxin. Am J Physiol Regul Integr Comp Physiol. 2011;301(2):R267–75.
Novak J, Danielson LA, Kerchner LJ, Sherwood OD, Ramirez RJ, Moalli PA, Conrad KP. Relaxin is essential for renal vasodilation during pregnancy in conscious rats. J Clin Invest. 2001;107(11):1469–75.
Debrah DO, Novak J, Matthews JE, Ramirez RJ, Shroff SG, Conrad KP. Relaxin is essential for systemic vasodilation and increased global arterial compliance during early pregnancy in conscious rats. Endocrinology. 2006;147(11):5126–31.
Smith MC, Murdoch AP, Danielson LA, Conrad KP, Davison JM. Relaxin has a role in establishing a renal response in pregnancy. Fertil Steril. 2006;86(1):253–5.
Rose BI, Brown SE. A review of the physiology behind letrozole applications in infertility: are current protocols optimal? J Assist Reprod Genet. 2020;37(9):2093–104.
Fauser BC, Van Heusden AM. Manipulation of human ovarian function: physiological concepts and clinical consequences. Endocr Rev. 1997;18(1):71–106.
Kristensen SG, Mamsen LS, Jeppesen JV, Bøtkjær JA, Pors SE, Borgbo T, Ernst E, Macklon KT, Andersen CY. Hallmarks of human small antral follicle development: implications for regulation of ovarian steroidogenesis and selection of the dominant follicle. Front Endocrinol (Lausanne). 2017;8:376.
Franks S, Hardy K. Androgen action in the ovary. Front Endocrinol (Lausanne). 2018;9:452.
Tavaniotou A, Albano C, Smitz J, Devroey P. Impact of ovarian stimulation on corpus luteum function and embryonic implantation. J Reprod Immunol. 2002;55(1-2):123–30.
Bülow NS, Skouby SO, Warzecha AK, Udengaard H, Andersen CY, Holt MD, Grøndahl ML, Nyboe Andersen A, Sopa N, Mikkelsen ALE, et al. Impact of letrozole co-treatment during ovarian stimulation with gonadotrophins for IVF: a multicentre, randomized, double-blinded placebo-controlled trial. Hum Reprod. 2022;37(2):309–21.
Miller PB, Parnell BA, Bushnell G, Tallman N, Forstein DA, Higdon HL 3rd, Kitawaki J, Lessey BA. Endometrial receptivity defects during IVF cycles with and without letrozole. Hum Reprod. 2012;27(3):881–8.
Ganesh A, Chauhan N, Das S, Chakravarty B, Chaudhury K. Endometrial receptivity markers in infertile women stimulated with letrozole compared with clomiphene citrate and natural cycles. Syst Biol Reprod Med. 2014;60(2):105–11.
Acknowledgements
The authors thank the medical workers in the research group at the Reproductive Hospital of Shandong University and also thank the information engineer for assembling the data.
Funding
This study was supported by the National Key Research and Development Program of China (2021YFC2700404).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The ethics committee at the Reproductive Hospital of Shandong University approved the study protocol.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 22 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Fu, X., Gao, S. et al. Letrozole use in vitrified single-blastocyst transfer cycles is associated with lower risk of large for gestational age infants in patients with polycystic ovary syndrome. J Assist Reprod Genet 40, 2885–2894 (2023). https://doi.org/10.1007/s10815-023-02956-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-023-02956-z