Abstract
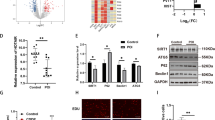
Primary ovarian insufficiency (POI) is a common condition leading to the pathological decline of ovarian function in women of reproductive age, resulting in amenorrhea, hypogonadism, and infertility. Biochemical premature ovarian insufficiency (bPOI) is an intermediate stage in the pathogenesis of POI in which the fertility of patients has been reduced. Previous studies suggest that granulosa cells (GCs) play an essential role in the pathogenesis of POI, but their pathogenetic mechanisms remain unclear. To further explore the potential pathophysiological mechanisms of GCs in POI, we constructed a molecular long non-coding RNA (lncRNA)-microRNA (miRNA)-messenger RNA (mRNA) network using GC expression data collected from biochemical premature ovarian failure (bPOI) patients in the GEO database. We discovered that the GCs of bPOI patients had differential expression of 131 mRNAs, 191 lncRNAs, and 28 miRNAs. By systematic network analysis, we identified six key genes, including SRSF1, PDIA5, NEURL1B, UNK, CELF2, and CFL2, and five hub miRNAs, namely hsa-miR-27a-3p, hsa-miR-24-3p, hsa-miR-22-3p, hsa-miR-129-5p, and hsa-miR-17-5p, and the results suggest that the expression of these key genes may be regulated by two hub miRNAs, hsa-miR-27a-3p and hsa-miR-17-5p. Additionally, a POI model in vitro was created to confirm the expression of a few important genes. In this study, we discovered a unique lncRNA-miRNA-mRNA network based on the ceRNA mechanism in bPOI for the first time, and we screened important associated molecules, providing a partial theoretical foundation to better understand the pathogenesis of POI.







Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are available in the Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/) database (Accession Number: GSE135697, GSE100238).
References
Jiao X, Ke H, Qin Y, Chen ZJ. Molecular genetics of premature ovarian insufficiency. Trends Endocrinol Metab. 2018;29:795–807.
Lew R. Natural history of ovarian function including assessment of ovarian reserve and premature ovarian failure. Best Pract Res Clin Obstet Gynaecol. 2019;55:2–13.
Huhtaniemi I, Hovatta O, La Marca A, Livera G, Monniaux D, Persani L, et al. Advances in the molecular pathophysiology, genetics, and treatment of primary ovarian insufficiency. Trends Endocrinol Metab. 2018;29:400–19.
Tu J, Chen Y, Li Z, Yang H, Chen H, Yu Z. Long non-coding RNAs in ovarian granulosa cells. J Ovarian Res. 2020;13:63.
Pankiewicz K, Laudański P, Issat T. The Role of Noncoding RNA in the Pathophysiology and Treatment of Premature Ovarian Insufficiency. Int J Mol Sci. 2021;22(17):9336. https://doi.org/10.3390/ijms22179336
Veitia RA. Primary ovarian insufficiency, meiosis and DNA repair. Biomed J. 2020;43:115–23.
Liu P, Zhang X, Hu J, Cui L, Zhao S, Jiao X, et al. Dysregulated cytokine profile associated with biochemical premature ovarian insufficiency. Am J Reprod Immunol. 2020;84:e13292.
Wang X, Zhang X, Dang Y, Li D, Lu G, Chan WY, et al. Long noncoding RNA HCP5 participates in premature ovarian insufficiency by transcriptionally regulating MSH5 and DNA damage repair via YB1. Nucleic Acids Res. 2020;48:4480–91.
Zhang X, Dang Y, Liu R, Zhao S, Ma J, Qin Y. MicroRNA-127-5p impairs function of granulosa cells via HMGB2 gene in premature ovarian insufficiency. J Cell Physiol. 2020;235:8826–38.
Dahariya S, Paddibhatla I, Kumar S, Raghuwanshi S, Pallepati A, Gutti RK. Long non-coding RNA: classification, biogenesis and functions in blood cells. Mol Immunol. 2019;112:82–92.
Sanchez Calle A, Kawamura Y, Yamamoto Y, Takeshita F, Ochiya T. Emerging roles of long non-coding RNA in cancer. Cancer Sci. 2018;109:2093–100.
Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs. Cell. 2018;172:393–407.
Hu H, Jia Q, Xi J, Zhou B, Li Z. Integrated analysis of lncRNA, miRNA and mRNA reveals novel insights into the fertility regulation of large white sows. BMC Genomics. 2020;21:636.
Ren GL, Zhu J, Li J, Meng XM. Noncoding RNAs in acute kidney injury. J Cell Physiol. 2019;234:2266–76.
Niu ZS, Wang WH, Dong XN, Tian LM. Role of long noncoding RNA-mediated competing endogenous RNA regulatory network in hepatocellular carcinoma. World J Gastroenterol. 2020;26:4240–60.
Panni S, Lovering RC, Porras P, Orchard S. Non-coding RNA regulatory networks. Biochim Biophys Acta Gene Regul Mech. 2020;1863:194417.
Caponnetto A, Battaglia R, Ferrara C, Vento ME, Borzì P, Paradiso M, et al. Down-regulation of long non-coding RNAs in reproductive aging and analysis of the lncRNA-miRNA-mRNA networks in human cumulus cells. J Assist Reprod Genet. 2022;39:919–31.
Chen H, Cheng S, Xiong W, Tan X. The lncRNA-miRNA-mRNA ceRNA network in mural granulosa cells of patients with polycystic ovary syndrome: an analysis of Gene Expression Omnibus data. Ann Transl Med. 2021;9:1156.
Chin CH, Chen SH, Wu HH, Ho CW, Ko MT, Lin CY. cytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst Biol. 2014;8(Suppl 4):S11.
Nishi Y, Yanase T, Mu Y, Oba K, Ichino I, Saito M, et al. Establishment and characterization of a steroidogenic human granulosa-like tumor cell line, KGN, that expresses functional follicle-stimulating hormone receptor. Endocrinology. 2001;142:437–45.
Wu Y, Ma C, Zhao H, Zhou Y, Chen Z, Wang L. Alleviation of endoplasmic reticulum stress protects against cisplatin-induced ovarian damage. Reprod Biol Endocrinol. 2018;16:85.
Webber L, Davies M, Anderson R, Bartlett J, Braat D, Cartwright B, et al. ESHRE guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016;31:926–37.
Laven JS. Primary ovarian insufficiency. Semin Reprod Med. 2016;34:230–4.
Dang Y, Wang X, Hao Y, Zhang X, Zhao S, Ma J, et al. MicroRNA-379-5p is associate with biochemical premature ovarian insufficiency through PARP1 and XRCC6. Cell Death Dis. 2018;9:106.
Vishnoi A, Rani S. MiRNA biogenesis and regulation of diseases: an overview. Methods Mol Biol. 2017;1509:1–10.
Bouckenheimer J, Fauque P, Lecellier CH, Bruno C, Commes T, Lemaître JM, et al. Differential long non-coding RNA expression profiles in human oocytes and cumulus cells. Sci Rep. 2018;8:2202.
Guo Y, Sun J, Lai D. Role of microRNAs in premature ovarian insufficiency. Reprod Biol Endocrinol. 2017;15:38.
Li D, Wang X, Li G, Dang Y, Zhao S, Qin Y. LncRNA ZNF674-AS1 regulates granulosa cell glycolysis and proliferation by interacting with ALDOA. Cell Death Discov. 2021;7:107.
Malmuthuge N, Guan LL. Noncoding RNAs: regulatory molecules of host-microbiome crosstalk. Trends Microbiol. 2021;29:713–24.
Rinn JL, Chang HY. Long noncoding RNAs: molecular modalities to organismal functions. Annu Rev Biochem. 2020;89:283–308.
Zhang H, Lu B. The Roles of ceRNAs-mediated autophagy in cancer chemoresistance and metastasis. Cancers (Basel). 2020;12(10):2926. https://doi.org/10.3390/cancers12102926
Liao J, Wang J, Liu Y, Li J, Duan L. Transcriptome sequencing of lncRNA, miRNA, mRNA and interaction network constructing in coronary heart disease. BMC Med Genomics. 2019;12:124.
Qi X, Lin Y, Chen J, Shen B. Decoding competing endogenous RNA networks for cancer biomarker discovery. Brief Bioinform. 2020;21:441–57.
Liu H, Gong Z, Li K, Zhang Q, Xu Z, Xu Y. SRPK1/2 and PP1α exert opposite functions by modulating SRSF1-guided MKNK2 alternative splicing in colon adenocarcinoma. J Exp Clin Cancer Res. 2021;40:75.
Wang H, Zhang Y, Zhang J, Du X, Li Q, Pan Z. circSLC41A1 resists porcine granulosa cell apoptosis and follicular atresia by promoting SRSF1 through miR-9820-5p sponging. Int J Mol Sci. 2022;23(3):1509. https://doi.org/10.3390/ijms23031509
Zhang H, He J, Dai Z, Wang Z, Liang X, He F, et al. PDIA5 is correlated with immune infiltration and predicts poor prognosis in gliomas. Front Immunol. 2021;12:628966.
Casali PG, Blay JY, Abecassis N, Bajpai J, Bauer S, Biagini R, et al. Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:20–33.
Yeung YT, Fan S, Lu B, Yin S, Yang S, Nie W, et al. CELF2 suppresses non-small cell lung carcinoma growth by inhibiting the PREX2-PTEN interaction. Carcinogenesis. 2020;41:377–89.
Xie SC, Zhang JQ, Jiang XL, Hua YY, Xie SW, Qin YA, et al. LncRNA CRNDE facilitates epigenetic suppression of CELF2 and LATS2 to promote proliferation, migration and chemoresistance in hepatocellular carcinoma. Cell Death Dis. 2020;11:676.
Piqué L, Martinez de Paz A, Piñeyro D, Martínez-Cardús A, Castro de Moura M, Llinàs-Arias P, et al. Epigenetic inactivation of the splicing RNA-binding protein CELF2 in human breast cancer. Oncogene. 2019;38:7106–12.
Liu J, Liu Z, Zhang X, Yan Y, Shao S, Yao D, et al. Aberrant methylation and microRNA-target regulation are associated with downregulated NEURL1B: a diagnostic and prognostic target in colon cancer. Cancer Cell Int. 2020;20:342.
Vollrath B, Pudney J, Asa S, Leder P, Fitzgerald K. Isolation of a murine homologue of the Drosophila neuralized gene, a gene required for axonemal integrity in spermatozoa and terminal maturation of the mammary gland. Mol Cell Biol. 2001;21:7481–94.
Nguyen MT, Min KH, Lee W. Palmitic acid-induced miR-429-3p impairs myoblast differentiation by downregulating CFL2. Int J Mol Sci. 2021;22(20):10972. https://doi.org/10.3390/ijms222010972
Nguyen MT, Lee W. Role of MiR-325-3p in the regulation of CFL2 and myogenic differentiation of C2C12 myoblasts. Cells. 2021;10(10):2725. https://doi.org/10.3390/cells10102725
Fattori F, Fiorillo C, Rodolico C, Tasca G, Verardo M, Bellacchio E, et al. Expanding the histopathological spectrum of CFL2-related myopathies. Clin Genet. 2018;93:1234–9.
Dang Y, Zhao S, Qin Y, Han T, Li W, Chen ZJ. MicroRNA-22-3p is down-regulated in the plasma of Han Chinese patients with premature ovarian failure. Fertil Steril. 2015;103:802–807.e801.
Ding C, Zhu L, Shen H, Lu J, Zou Q, Huang C, et al. Exosomal miRNA-17-5p derived from human umbilical cord mesenchymal stem cells improves ovarian function in premature ovarian insufficiency by regulating SIRT7. Stem Cells. 2020;38:1137–48.
Acknowledgements
This work was supported by the Department of Obstetrics and Gynecology, Shanghai Tenth People’s Hospital, and Tongji University. The results published here are in whole or partly based on data obtained from the Gene expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/).
Funding
This project was supported by the National Nature Science Foundation of China (NO. 31900522).
Author information
Authors and Affiliations
Contributions
BL and LL contributed equally to this work. BL and LL contributed to the idea of the manuscript, completed the experiment, analyzed the data, and wrote this manuscript. BL and LL completed the revision of the manuscript. ZS, CW, JZ, and LW helped interpret the data. ZC and SL conceived the study. All authors read and approved the manuscript. BL and LL contributed equally to this work.
Corresponding authors
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Written informed consent was obtained from all participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, B., Liu, L., Sulaiman, Z. et al. Comprehensive analysis of lncRNA-miRNA-mRNA ceRNA network and key genes in granulosa cells of patients with biochemical primary ovarian insufficiency. J Assist Reprod Genet 41, 15–29 (2024). https://doi.org/10.1007/s10815-023-02937-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-023-02937-2