Abstract
Purpose
This study aims to achieve the methodological improvement of rescue IVM by predicting germinal vesicle breakdown (GVBD) and optimizing the timing of ICSI.
Methods
Time lapse analysis was performed retrospectively to evaluated the relationship between the presence of AC around the nucleoli and GVBD. To find the optimal timing of ICSI, the time from the initiation of the first polar body extrusion to ICSI were measured, and the rates of fertilization at each point were calculated.
Results
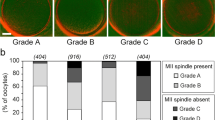
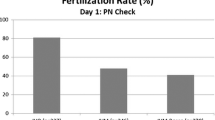
The GVBD rate of GV stage oocytes with AC around the nucleoli was significantly higher than that of GV stage oocytes without AC. The GV stage oocytes required more time for nuclear maturation after polar body extrusion than MI oocytes, with GV stage oocytes taking 400–600 min from polar body extrusion to the optimal timing of ICSI, while the MI stage oocytes took 200–400 min. The GV stage oocytes resulted in the birth of healthy babies with the appropriate timing of ICSI.
Conclusion
It was found that GV stage oocytes with AC around nucleoli can initiate GVBD and reach the MII stage with a high rate, and that GV stage oocytes required more time than MI stage oocytes to reach the optimal timing of ICSI. Considering these factors, ART laboratories may employ immature GV stage oocytes in routine ART procedures rather than discarding them.




Similar content being viewed by others
Data availability
The data presented in this study are available on request from the corresponding author.
References
Cha KY, Chian RC. Maturation in vitro of immature human oocytes for clinical use. Hum Reprod Update. 1998;4:103–20. https://doi.org/10.1093/humupd/4.2.103.
De Vos A, Van de Velde H, Joris H, Van Steirteghem A. In-vitro matured metaphase-I oocytes have a lower fertilization rate but similar embryo quality as mature metaphase-II oocytes after intracytoplasmic sperm injection. Hum Reprod. 1999;14:1859–63. https://doi.org/10.1093/humrep/14.7.1859.
Jee BC, Han SH, Moon JH, Suh CS, Kim SH. Influence of well defined protein source on in vitro maturation of human oocyte: human follicular fluid versus human serum albumin. Fertil Steril. 2008;89:348–52. https://doi.org/10.1016/j.fertnstert.2007.02.052.
Huddleston HG, Jackson KV, Doyle JO, Racowsky C. hMG increases the yield of mature oocytes and excellent-quality embryos in patients with a previous cycle having a high incidence of oocyte. Fertil Steril. 2009;92(3):946–9. https://doi.org/10.1016/j.fertnstert.2009.02.039.
Edirisinghe WR, Junk SM, Matson PL, Yovich JL. Birth from cryopreserved embryos following in-vitro maturation of oocytes and intracytoplasmic sperm injection. Hum Reprod. 1997;12(5):1056–8. https://doi.org/10.1093/humrep/12.5.1056.
Son W-Y, Lee S-Y, Lim J-H. Fertilization, cleavage and blastocyst development according to the maturation timing of oocytes in in vitro maturation cycles. Hum Reprod. 2005;20(11):3204–7. https://doi.org/10.1093/humrep/dei195.
Otsuki J, Momma Y, Takahashi K, Miyakura S, Nagai Y. Timed IVM followed by ICSI in a patient with immature ovarian oocytes. Reprod Biomed Online. 2006;13(1):101–3. https://doi.org/10.1016/s1472-6483(10)62022-6.
Nagy ZP, Cecile J, Liu J, Loccufier A, Devroey P, Steirteghem AV. Pregnancy and birth after intracytoplasmic sperm injection of in vitro matured germinal-vesicle stage oocytes: case report. Fertil Steril. 1996;65(5):1047–50. https://doi.org/10.1016/S0015-0282(16)58285-5.
Escrich L, Galiana Y, Grau N, Insua F, Soler N, Pellicer A, Escribá MJ. Do immature and mature sibling oocytes recovered from stimulated cycles have the same reproductive potential? Reprod Biomed Online. 2018;37(6):667–76. https://doi.org/10.1016/j.rbmo.2018.08.023.
Vlaisavljević V, Kovac V, Sajko MC. Impact of insulin resistance on the developmental potential of immature oocytes retrieved from human chorionic gonadotropin-primed women with polycystic ovary syndrome undergoing in vitro maturation. Fertil Steril. 2009;91(3):957–9. https://doi.org/10.1016/j.fertnstert.2007.12.062.
Tannus S, Hatirnaz S, Tan J, Ata B, Tan SL, Hatirnaz E, Kenat-Pektas M, Dahan MH. Predictive factors for live birth after in vitro maturation of oocytes in women with polycystic ovary syndrome. Arch Gynecol Obstet. 2018;297(1):199–204. https://doi.org/10.1007/s00404-017-4561-z.
Madkour A, Bouamoud N, Kaarouch I, Louanjli N, Saadani B, Assou S, Aboulmaouahib S, Sefrioui O, Amzazi S, Copin H, Benkhalifa M. Follicular fluid and supernatant from cultured cumulus-granulosa cells improve in vitro maturation in patients with polycystic ovarian syndrome. Fertil Steril. 2018;110(4):710–9. https://doi.org/10.1016/j.fertnstert.2018.04.038.
Le Du A, Kadoch IJ, Bourcigaux N, Doumerc S, Bourrier M-C, Chevalier N, Fanchin R, Chian R-C, Tachdjian G, Frydman R, Frydman N. In vitro oocyte maturation for the treatment of infertility associated with polycystic ovarian syndrome: the French experience. Hum Reprod. 2005;20(2):420–4. https://doi.org/10.1093/humrep/deh603.
Sánchez F, Lolicato F, Romero S, De Vos M, Van Ranst H, Verheyen G, Anckaert E, Smitz JEJ. An improved IVM method for cumulus-oocyte complexes from small follicles in polycystic ovary syndrome patients enhances oocyte competence and embryo yield. Hum Reprod. 2017;32(10):2056–68. https://doi.org/10.1093/humrep/dex262.
Ranganath A, Appaneravanda LC, Gerstl B, Math NT, Menon J, Gunasheela D. A Study to Find Optimal Intra-cytoplasmic Sperm Injection Timing of Oocytes Matured from Germinal Vesicle in in Vitro Maturation Cycles Using a Time Lapse System. Hum Reprod. 2021;14(4):415–21. https://doi.org/10.4103/jhrs.jhrs_130_21.
Kim B-K, Lee S-C, Kim K-J, Han C-H, Kim J-H. In vitro maturation, fertilization, and development of human germinal vesicle oocytes collected from stimulated cycles. Fertil Steril. 2000;74(6):1153–8. https://doi.org/10.1016/s0015-0282(00)01617-4.
De Vos M, Grynberg M, Ho TM, Yuan Y, Albertini DF, Gilchrist RB. Perspectives on the development and future of oocyte IVM in clinical practice. J Assist Reprod Genet. 2021;38(6):1265–80. https://doi.org/10.1007/s10815-021-02263-5.
Jie H, Zhao M, Alqawasmeh OAM, Chan CPS, Lee TL, Li T, Chan DYL. In vitro rescue immature oocytes - a literature review. Fertil Steril. 2021;1–20. https://www.tandfonline.com/doi/full/10.1080/14647273.2021.1876932. Accessed 16 June 2023
Jones GM, Cram DS, Song B, Magli MC, Gianaroli L, Lacham-Kaplan O, Findlay JK, Jenkin G, Trounson AO. Gene expression profiling of human oocytes following in vivo or in vitro maturation. Hum Reprod. 2008;23:1138–44. https://doi.org/10.1093/humrep/den085.
Otsuki J, Nagai Y. A phase of chromosome aggregation during meiosis in human oocytes. Reprod Biomed Online. 2007;15(2):191–7. https://doi.org/10.1016/s1472-6483(10)60708-0.
Holubcova Z, Blayney M, Elder K, Schuh M. Error-prone chromosome-mediated spindle assembly favors chromosome segregation defects in human oocytes. Science. 2015;348(6239):1143–7. https://doi.org/10.1126/science.aaa9529.
Harasimov K, Uraji J, Mönnich EU, Holubcová Z, Elder K, Blayney M, Schuh M. Actin-driven chromosome clustering facilitates fast and complete chromosome capture in mammalian oocytes. Nat Cell Biol. 2023;25(3):439–52. https://doi.org/10.1038/s41556-022-01082-9.
Salimov D, Lopata A, Nagai Y, Lisovskaya T, Portnov I, Miwa A, Otuki J. In human oocytes the aggregation of chromosomes around the nucleolus is associated with the initiation and completion of metiotic maturation. abstract of ASRM. 2014. https://www.fertstert.org/article/S0015-0282(14)01033-4. Accessed 2 May 2023
Parfenov V, Potchukalina G, Dudina L, Kostyuchek D, Gruzova M. Human antral follicles: oocyte nucleus and the karyosphere formation (electron microscopic and autoradiographic data). Gamete Res. 1989;22(2):219–31. https://doi.org/10.1002/mrd.1120220209.
Mattson BA, Albertini DE. Oogenesis: chromatin and microtubule dynamics during meiotic prophase. Mol Reprod Dev. 1990;25(4):374–83. https://doi.org/10.1002/mrd.1080250411.
Debey P, Szöllösi MS, Szöllösi D, Vautier D, Girousse A, Besombes D. Competent mouse oocytes isolated from antral follicles exhibit different chromatin organization and follow different maturation dynamics. Mol Reprod Dev. 1993;36(1):59–74. https://doi.org/10.1002/mrd.1080360110.
Zuccotti M, Piccinelli A, Giorgi Rossi P, Garagna S, Redi CA. Chromatin organization during mouse oocyte growth. Mol Reprod Dev. 1995;41(4):479–85. https://doi.org/10.1002/mrd.1080410410.
Zuccotti M, Giorgi Rossi P, Martinez A, Garagna S, Forabosco A, Redi CA. Meiotic and developmental competence of mouse antral oocytes. Biol Reprod. 1998;58(3):700–4. https://doi.org/10.1095/biolreprod58.3.700.
Christians E, Boiani M, Garagna S, Dessy C, Redi CA, Renard JP, Zuccotti M. Gene expression and chromatin organization during mouse oocyte growth. Dev Biol. 1999;207(1):76–85. https://doi.org/10.1006/dbio.1998.9157.
Montag M, Schimming T, van der Ven H. Spindle imaging in human oocytes: the impact of the meiotic cell cycle. Reprod Biomed Online. 2006;12(4):442–6. https://doi.org/10.1016/s1472-6483(10)61996-7.
Yang Q, Zhu L, Wang M, Huang Bo, Li Z, Juan Hu, Xi Q, Liu J, Jin L. Analysis of maturation dynamics and developmental competence of in vitro matured oocytes under time-lapse monitoring. Reprod Biol Endocrinol. 2021;19(1):183. https://doi.org/10.1186/s12958-021-00868-0.
Nogueira D, Staessen C, Van de Velde H, Van Steirteghem A. Nuclear status and cytogenetics of embryos derived from in vitro-matured oocytes. Fertil Steril. 2000;74:295–8. https://doi.org/10.1016/s0015-0282(00)00642-7.
Conti M, Franciosi F. Acquisition of oocyte competence to develop as an embryo: integrated nuclear and cytoplasmic events. Hum Reprod Update. 2018;24(3):245–66. https://doi.org/10.1093/humupd/dmx040.
Acknowledgements
The authors would like to thank all of the lab members of assisted reproductive technology unit of Okayama University for the productive discussions.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception after designed by Junko Otsuki. Material preparation, data collection and analysis were performed by Hayato Asama, Daigaku Kamibayashi, Atsuko Hashizume and Yasuhito Michikura. The first draft of the manuscript was written by Hayato Asama and Junko Otsuki and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Okayama University Institutional Review Board (approval no. K2205-011).
Conflict of interest
All of the authors declare that they have no conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Asama, H., Kamibayashi, D., Hashizume, A. et al. Focusing on the accumulation of chromatin/chromosomes around nucleoli and optimizing the timing of ICSI to facilitate the rescue in vitro maturation of denuded GV stage oocytes. J Assist Reprod Genet 40, 2557–2564 (2023). https://doi.org/10.1007/s10815-023-02921-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-023-02921-w