Abstract
Purpose
Evaluate which factors are involved in the increased rate of mosaicism in embryos.
Methods
A systematic review and meta-analysis was performed. After an exhaustive search of the literature, a total of seven papers were included in the analysis. In addition, data collected from IVF cycles performed in our fertility clinic were also analysed. Day of biopsy, embryo quality, maternal and paternal age and seminal quality were the chosen factors to be studied.
Results
The results of the meta-analysis show that neither embryo quality nor seminal quality were related to mosaic embryo rate (OR: 1.09; 95% CI: 0.94–1.28 and OR: 1.10; 95% CI: 0.87–1.37, respectively). A positive association was observed for the variable “biopsy day” with embryos biopsied at day 6 or 7 having the highest rate of mosaicism (OR: 1.06; 95% CI: 1.01–1.11). In opposite to what happens with aneuploidy rate, which increases with maternal age, embryo mosaicism is higher in younger women (<34 years) rather than in older ones (≥34 years) (OR: 0.95; 95% CI: 0.92–0.98). However, for the “paternal age” factor, no association with mosaicism was found (OR: 1.04; 95% CI: 0.90–1.21).
Conclusions
With the present study, we can conclude that the factors related to the presence of mosaicism in embryos are the embryo biopsy day and maternal age. The rest of the studied factors showed no significant relationship with mosaicism. These results are of great importance as knowing the possible causes leading to mosaicism helps to improve the clinical results of reproductive treatments.


Similar content being viewed by others
Data Availability
The authors confirm that the data supporting this article are available under request.
References
Greco E, Minasi MG, Fiorentino F. Healthy babies after intrauterine transfer of mosaic aneuploid blastocysts. N Engl J Med. 2015;373(21):2089–90. https://doi.org/10.1056/NEJMc1500421.
Webster A, Schuh M. Mechanisms of aneuploidy in human eggs. Trends Cell Biol. 2017;27:55–68.
Taylor TH, Gitlin SA, Patrick JL, Crain JL, Wilson JM, Griffin DK. The origin, mechanisms, incidence and clinical consequences of chromosomal mosaicism in humans. Hum Reprod Update. 2014;20(4):571–81. https://doi.org/10.1093/humupd/dmu016.
Alksere B, Grinfelde I, Kornejeva L, Dzalbs A, Vedmedovska N, Kovalova I, Conka U, Andersone S, Krasucka S, Blumberga A, Berzina D, Fodina V. The outcomes after transfers of embryos with chromosomal mosaicism: a single reproductive medicine center experience at IVF Riga clinic. Gynecol Endocrinol. 2020;36(sup1):53–7. https://doi.org/10.1080/09513590.2020.1816719.
Popovic M, Dhaenens L, Boel A, Menten B, Heindryckx B. Chromosomal mosaicism in human blastocysts: the ultimate diagnostic dilemma. Hum Reprod Update. 2020;26:313–34.
Zhang YX, Chen JJ, Nabu S, Yeung QSY, Li Y, Tan JH, Suksalak W, Chanchamroen S, Quangkananurug W, Wong PS, Chung JPW, Choy KW. The pregnancy outcome of mosaic embryo transfer: a prospective multicenter study and meta-analysis. Genes. 2020;11(9):973. https://doi.org/10.3390/genes11090973.
Coll L, Parriego M, Mateo S, García-Monclús S, Rodríguez I, Boada M, Coroleu B, Polyzos NP, Vidal F, Veiga A. Prevalence, types and possible factors influencing mosaicism in IVF blastocysts: results from a single setting. Reprod Biomed Online. 2021;42(1):55–65. https://doi.org/10.1016/j.rbmo.2020.09.025.
Cram DS, Leigh D, Handyside A, Rechitsky L, Xu K, Harton G, Grifo J, Rubio C, Fragouli E, Kahraman S, Forman E, Katz-Jaffe M, Tempest H, Thornhill A, Strom C, Escudero T, Qiao J, Munne S, Simpson JL, Kuliev A. PGDIS position statement on the transfer of Mosaic Embryos 2019. Reprod Biomed Online. 2019;39(Suppl 1):e1–4. https://doi.org/10.1016/j.rbmo.2019.06.012.
Swain JE. Controversies in ART: can the IVF laboratory influence preimplantation embryo aneuploidy? Reprod Biomed Online. 2019;39:599–607. https://doi.org/10.1016/j.rbmo.2019.06.009.
Capalbo A, Poli M, Rienzi L, Girardi L, Patassini C, Fabiani M, Cimadomo D, Benini F, Farcomeni A, Cuzzi J, Rubio C, Albani E, Sacchi L, Vaiarelli A, Figliuzzi M, Findikli N, Coban O, Boynukalin FK, Vogel I, et al. Mosaic human preimplantation embryos and their developmental potential in a prospective, non-selection clinical trial. Am J Hum Genet. 2021;108(12):2238–47. https://doi.org/10.1016/j.ajhg.2021.11.002.
Martín Á, Rodrigo L, Beltrán D, Meseguer M, Rubio C, Mercader A, de Los Santos MJ. The morphokinetic signature of mosaic embryos: evidence in support of their own genetic identity. Fertil Steril. 2021;116(1):165–73. https://doi.org/10.1016/j.fertnstert.2020.12.031.
Lledó B, Morales R, Ortiz JA, Blanca H, Ten J, Llácer J, Bernabeu R. Implantation potential of mosaic embryos. Syst Biol Reprod Med. 2017;63(3):206–8. https://doi.org/10.1080/19396368.2017.1296045.
Morbeck DE, Baumann NA, Oglesbee D. Composition of single-step media used for human embryo culture. Fertil Steril. 2017:107. https://doi.org/10.1016/j.fertnstert.2017.01.007.
Katz-Jaffe M, Parks J, McReynolds S, Henry L, Schoolcraft WB. Chromosomal mosaicism is impacted by compromised embryo culture conditions. Fertil Steril. 2018;110:e431. https://doi.org/10.1016/j.fertnstert.2018.08.037.
Monahan D, Harton G, Griffin D, Angle M, Smikle C. Clinical comparison of two PGT-A platforms utilizing different thresholds to determine ploidy status. Reprod Biomed Online. 2019;39:e27–e28.2. https://doi.org/10.1016/j.rbmo.2019.04.055.
Rodrigo L, Clemente-Císcar M, Campos-Galindo I, Peinado V, Simón C, Rubio C. Characteristics of the IVF cycle that contribute to the incidence of mosaicism. Genes. 2020;11(10):1151. https://doi.org/10.3390/genes11101151.
ESHRE Working Group on Chromosomal Mosaicism and others. ESHRE survey results and good practice recommendations on managing chromosomal mosaicism. Human Reproduction Open. 2022(4):hoac044. https://doi.org/10.1093/hropen/hoac044.
Victor AR, Tyndall JC, Brake AJ, Lepkowsky LT, Murphy AE, Griffin DK, McCoy RC, Barnes FL, Zouves CG, Viotti M. One hundred mosaic embryos transferred prospectively in a single clinic: exploring when and why they result in healthy pregnancies. Fertil Steril. 2019;111(2):280–93. https://doi.org/10.1016/j.fertnstert.2018.10.019.
Zore T, Kroener LL, Wang CM, Liu L, Buyalos R, Hubert G, Shamonki M. Transfer of embryos with segmental mosaicism is associated with a significant reduction in live-birth rate. Fertil Steril. 2019;111:69–76.
Zhang L, Wei D, Zhu Y, Gao Y, Yan J, Chen ZJ. Rates of live birth after mosaic embryo transfer compared with euploid embryo transfer. J Assist Reprod Genet. 2019;36(1):165–72. https://doi.org/10.1007/s10815-018-1322-2.
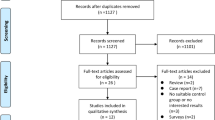
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9.
Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26:1270–83.
Ardoy M, Caderón G, Cuadros J, Figueroa MJ, Herrer R, Moreno JM, et al. Criterios ASEBIR de valoración morfológica de oocitos, embriones tempranos y blastocistos humanos. Cuadernos de Embriología Clínica. 2008;II:1–59.
Björndahl L, Kirkman Brown J. The sixth edition of the WHO Laboratory Manual for the Examination and Processing of Human Semen: ensuring quality and standardization in a basic examination of human ejaculates. Fertil Steril. 2022;117(2):246–51.
Domenech JM. Macro !MAR for SPSS Statistics. Meta-Analysis OR, RR, RD, IR, ID, B & MD Combined [computer program].V2012.03.23. Bellaterra: Universitat Autònoma de Barcelona; 2012.
Minasi MG, Colasante A, Riccio T, Ruberti A, Casciani V, Scarselli F, Spinella F, Fiorentino F, Varricchio MT, Greco E. Correlation between aneuploidy, standard morphology evaluation and morphokinetic development in 1730 biopsied blastocysts: a consecutive case series study. Hum Reprod. 2016;31(10):2245–54. https://doi.org/10.1093/humrep/dew183.
Capalbo A, Rienzi L, Cimadomo D, Maggiulli R, Elliott T, Wright G, Nagy ZP, Ubaldi FM. Correlation between standard blastocyst morphology, euploidy and implantation: an observational study in two centers involving 956 screened blastocysts. Hum Reprod. 2014;29(6):1173–81. https://doi.org/10.1093/humrep/deu033.
Ortiz JA, Morales R, Lledó B, Vicente JA, González J, García-Hernández EM, Cascales A, Ten J, Bernabeu A, Bernabeu R. Application of machine learning to predict aneuploidy and mosaicism in embryos from in vitro fertilization cycles. AJOG Glob Rep. 2022;2(4):100103. https://doi.org/10.1016/j.xagr.2022.100103.
Huang QX, Wang ZH, Huang WJ, Mao LH, Lin CL, Chen GY, Wang CX, Chen ZB, Lin YL, He LY, Liu Y. Factors influencing mosaicism: a retrospective analysis. Reprod Biomed Online. 2022;45(3):491–500. https://doi.org/10.1016/j.rbmo.2022.04.020.
ESHRE Capri Workshop Group. Fertility and ageing. Hum Reprod Update. 2005;11:261–76.
Babariya D, Fragouli E, Alfarawati S, Spath K, Wells D. The incidence and origin of segmental aneuploidy in human oocytes and preimplantation embryos. Hum Reprod. 2017;32(12):2549–60. https://doi.org/10.1093/humrep/dex324.
Barash OO, Hinckley MD, Rosenbluth EM, Ivani KA, Weckstein LN. High gonadotropin dosage does not affect euploidy and pregnancy rates in IVF PGS cycles with single embryo transfer. Hum Reprod. 2017;32(11):2209–17. https://doi.org/10.1093/humrep/dex299.
Munné S, Cohen J. Advanced maternal age patients benefit from preimplantation genetic diagnosis of aneuploidy. Fertil Steril. 2017;107(5):1145–6. https://doi.org/10.1016/j.fertnstert.2017.03.015.
Nakhuda G, Jing C, Butler R, Guimond C, Hitkari J, Taylor E, Tallon N, Yuzpe A. Frequencies of chromosome-specific mosaicisms in trophoectoderm biopsies detected by next-generation sequencing. Fertil Steril. 2018;109(5):857–65. https://doi.org/10.1016/j.fertnstert.2018.01.011.
Munné S, Grifo J, Wells D. Mosaicism: “survival of the fittest” versus “no embryo left behind”. Fertil Steril. 2016;105(5):1146–9. https://doi.org/10.1016/j.fertnstert.2016.01.016.
Keefe DL. Telomeres and genomic instability during early development. Eur J Med Genet. 2020;63(2):103638. https://doi.org/10.1016/j.ejmg.2019.03.002.
Keefe DL, Liu L. Telomeres and reproductive aging. Reprod Fertil Dev. 2009;21(1):10–4. https://doi.org/10.1071/rd08229.
Blackburn EH. Structure and function of telomeres. Nature. 1991;350(6319):569–73. https://doi.org/10.1038/350569a0.
Carrasquillo RJ, Kohn TP, Cinnioglu C, Rubio C, Simon C, Ramasamy R, Al-Asmar N. Advanced paternal age does not affect embryo aneuploidy following blastocyst biopsy in egg donor cycles. J Assist Reprod Genet. 2019;36(10):2039–45. https://doi.org/10.1007/s10815-019-01549-z.
Dviri M, Madjunkova S, Koziarz A, Antes R, Abramov R, Mashiach J, Moskovtsev S, Kuznyetsova I, Librach C. Is there a correlation between paternal age and aneuploidy rate? An analysis of 3,118 embryos derived from young egg donors. Fertil Steril. 2020;114(2):293–300. https://doi.org/10.1016/j.fertnstert.2020.03.034.
Pino V, Sanz A, Valdés N, Crosby J, Mackenna A. The effects of aging on semen parameters and sperm DNA fragmentation. JBRA Assist Reprod. 2020;24(1):82–6. https://doi.org/10.5935/1518-0557.20190058.
Tarozzi N, Nadalini M, Lagalla C, Coticchio G, Zacà C, Borini A. Male factor infertility impacts the rate of mosaic blastocysts in cycles of preimplantation genetic testing for aneuploidy. J Assist Reprod Genet. 2019;36(10):2047–55. https://doi.org/10.1007/s10815-019-01584-w.
Kahraman S, Sahin Y, Yelke H, Kumtepe Y, Tufekci MA, Yapan CC, Yesil M, Cetinkaya M. High rates of aneuploidy, mosaicism and abnormal morphokinetic development in cases with low sperm concentration. J Assist Reprod Genet. 2020;37(3):629–40. https://doi.org/10.1007/s10815-019-01673-w.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 18 kb)
Appendix. Systematic review search strategies
Appendix. Systematic review search strategies
PubMed—(“semen quality”[Title/Abstract] OR “embryo”[Title/Abstract] OR “preimplantation diagnosis”[Title/Abstract] OR “blastocyst”[Title/Abstract] OR “blastocysts”[Title/Abstract]) AND (“Mosaicism”[Title/Abstract] OR “embryo mosaicism”[Title/Abstract]) Filters: from 2016 to 2022.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cascales, A., Morales, R., Castro, A. et al. Factors associated with embryo mosaicism: a systematic review and meta-analysis. J Assist Reprod Genet 40, 2317–2324 (2023). https://doi.org/10.1007/s10815-023-02914-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-023-02914-9