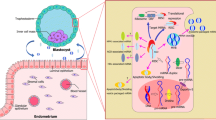
Abstract
Embryo implantation is a critical process for achieving a successful pregnancy and live birth. The proper implantation must have a synchronized interaction between blastocyst and a receptive endometrium. Many genes are involved in the modulation of precise molecular events during implantation. MicroRNAs (miRNAs) have been extensively reported as gene regulatory molecules on post-transcriptional levels involved in various biological processes such as gametogenesis, embryogenesis, and the quality of sperm, oocyte, and embryos. A plethora of evidence has demonstrated critical roles for miRNAs in regulating genes involved in the implantation process; hence, dysregulation of miRNAs could be associated with significant impairments in implantation, such as recurrent implantation failure. In addition to the indispensable role of miRNAs in the intracellular control of gene expression, they can also be secreted into extracellular fluid and circulation. Therefore, miRNAs in body fluids and blood may be exploited as non-invasive diagnostic biomarkers for different pathological and physiological conditions. Recently, several studies have focused on the discovery of miRNAs function in the implantation process by appraising miRNAs and their target genes in human embryos, endometrial tissue, and cell culture models. Moreover, it was revealed that there could be a significant association between endometrial receptivity or implantation status and the expression of miRNAs in human body fluids, reinforcing their role as non-invasive biomarkers. In the current work, we reviewed the studies concerning the role of intracellular and extracellular miRNAs in human implantation and the influence of their dysregulation on implantation disorders.


Similar content being viewed by others
References
Davidson LM, Coward K. Molecular mechanisms of membrane interaction at implantation. Birth Defects Res C Embryo Today. 2016;108(1):19–32.
Kim S-M, Kim J-S. A review of mechanisms of implantation. Dev Reprod. 2017;21(4):351.
Finch ML, Marquardt JU, Yeoh GC, Callus BA. Regulation of microRNAs and their role in liver development, regeneration and disease. Int J Biochem Cell Biol. 2014;54:288–303.
Salilew-Wondim D, Gebremedhn S, Hoelker M, Tholen E, Hailay T, Tesfaye D. The role of micrornas in mammalian fertility: from gametogenesis to embryo implantation. Int J Mol Sci. 2020;21(2):585.
Machtinger R, Rodosthenous RS, Adir M, Mansour A, Racowsky C, Baccarelli AA, et al. Extracellular microRNAs in follicular fluid and their potential association with oocyte fertilization and embryo quality: an exploratory study. J Assist Reprod Genet. 2017;34(4):525.
Alves MBR, Celeghini ECC, Belleannée C. From sperm motility to sperm-borne microRNA signatures: new approaches to predict male fertility potential. Front Cell Dev Biol. 2020;8:791.
Paul AB, Sadek ST, Mahesan AM. The role of microRNAs in human embryo implantation: a review. J Assist Reprod Genet. 2019;36(2):179–87.
Zhou W, Dimitriadis E. Secreted MicroRNA to predict embryo implantation outcome: from research to clinical diagnostic application. Front Cell Dev Biol. 2020;8:939.
Galliano D, Pellicer A. MicroRNA and implantation. Fertil Steril. 2014;101(6):1531–44.
Liang J, Wang S, Wang Z. Role of microRNAs in embryo implantation. Reprod Biol endocrinol. 2017;15(1):90.
Shekibi M, Heng S, Nie G. MicroRNAs in the regulation of endometrial receptivity for embryo implantation. Int J Mol Sci. 2022;23(11):6210.
Ochoa-Bernal MA, Fazleabas AT. Physiologic events of embryo implantation and decidualization in human and non-human primates. Int J Mol Sci. 2020;21(6):1973.
Evans J, Salamonsen LA, Winship A, Menkhorst E, Nie G, Gargett CE, et al. Fertile ground: human endometrial programming and lessons in health and disease. Nat Rev Endocrinol. 2016;12(11):654–67.
Wang B, Sheng JZ, He RH, Qian YL, Jin F, Huang HF. High expression of l-selectin ligand in secretory endometrium is associated with better endometrial receptivity and facilitates embryo implantation in human being. Am J Reprod Immunol. 2008;60(2):127–34.
Haller-Kikkatalo K, Altmaee S, Tagoma A, Uibo R, Salumets A, editors. Autoimmune activation toward embryo implantation is rare in immune-privileged human endometrium. Seminars in reproductive medicine. Thieme Medical Publishers; 2014.
Achache H, Revel A. Endometrial receptivity markers, the journey to successful embryo implantation. Hum Reprod Update. 2006;12(6):731–46.
Nachtigall MJ, Kliman HJ, Feinberg RF, Olive DL, Engin O, Arici A. The effect of leukemia inhibitory factor (LIF) on trophoblast differentiation: a potential role in human implantation. J Clin Endocrinol Metab. 1996;81(2):801–6.
Reddy K, Mangale SS. Integrin receptors: the dynamic modulators of endometrial function. Tissue Cell. 2003;35(4):260–73.
Ashary N, Tiwari A, Modi D. Embryo implantation: war in times of love. Endocrinology. 2018;159(2):1188–98.
Monroig PdC, Calin GA. MicroRNA and epigenetics: diagnostic and therapeutic opportunities. Curr Pathobiol Rep. 2013;1(1):43–52.
Makarova JA, Shkurnikov MU, Wicklein D, Lange T, Samatov TR, Turchinovich AA, et al. Intracellular and extracellular microRNA: an update on localization and biological role. Prog Histochem Cytochem. 2016;51(3–4):33–49.
Broughton JP, Lovci MT, Huang JL, Yeo GW, Pasquinelli AE. Pairing beyond the seed supports microRNA targeting specificity. Mol Cell. 2016;64(2):320–33.
O’Brien J, Hayder H, Zayed Y, Peng C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol. 2018;9:402.
Hammond SM. An overview of microRNAs. Adv Drug Deliv Rev. 2015;87:3–14.
Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18(10):997–1006.
Gallo A, Tandon M, Alevizos I, Illei GG. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS ONE. 2012;7(3): e30679.
da Silveira JC, Veeramachaneni DR, Winger QA, Carnevale EM, Bouma GJ. Cell-secreted vesicles in equine ovarian follicular fluid contain miRNAs and proteins: a possible new form of cell communication within the ovarian follicle. Biol Reprod. 2012;86(3):71, 1–10.
Weber JA, Baxter DH, Zhang S, Huang DY, How Huang K, Jen Lee M, et al. The microRNA spectrum in 12 body fluids. Clin Chem. 2010;56(11):1733–41.
Chen X, Liang H, Zhang J, Zen K, Zhang C-Y. Secreted microRNAs: a new form of intercellular communication. Trends Cell Biol. 2012;22(3):125–32.
Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15(8):509–24.
McCallie B, Schoolcraft WB, Katz-Jaffe MG. Aberration of blastocyst microRNA expression is associated with human infertility. Fertil Steril. 2010;93(7):2374–82.
Rosenbluth EM, Shelton DN, Sparks AE, Devor E, Christenson L, Van Voorhis BJ. MicroRNA expression in the human blastocyst. Fertil Steril. 2013;99(3):855–61. e3.
Zhang C, Lu X, Yang Y, Ayi N, Li L. Highly expressed microRNA-940 promotes early abortion by regulating placenta implantation by targeting ZNF672. Eur Rev Med Pharmacol Sci. 2019;23(7):2693–700.
Sun M, Chen H, Liu J, Tong C, Meng T. MicroRNA-34a inhibits human trophoblast cell invasion by targeting MYC. BMC Cell Biol. 2015;16(1):1–9.
Rosenbluth EM, Shelton DN, Wells LM, Sparks AE, Van Voorhis BJ. Human embryos secrete microRNAs into culture media—a potential biomarker for implantation. Fertil Steril. 2014;101(5):1493–500.
Cuman C, Van Sinderen M, Gantier MP, Rainczuk K, Sorby K, Rombauts L, et al. Human blastocyst secreted microRNA regulate endometrial epithelial cell adhesion. EBioMedicine. 2015;2(10):1528–35.
Borges Jr E, Setti AS, Braga DP, Geraldo MV, Figueira RdCS, Iaconelli Jr A. miR-142–3p as a biomarker of blastocyst implantation failure-a pilot study. JBRA Assist Reprod. 2016;20(4):200.
Capalbo A, Ubaldi FM, Cimadomo D, Noli L, Khalaf Y, Farcomeni A, et al. MicroRNAs in spent blastocyst culture medium are derived from trophectoderm cells and can be explored for human embryo reproductive competence assessment. Fertil Steril. 2016;105(1):225–35. e3.
Abu-Halima M, Khaizaran ZA, Ayesh BM, Fischer U, Khaizaran SA, Al-Battah F, et al. MicroRNAs in combined spent culture media and sperm are associated with embryo quality and pregnancy outcome. Fertil Steril. 2020;113(5):970–80. e2.
Ata B, Kalafat E, Somigliana E. A new definition of recurrent implantation failure on the basis of anticipated blastocyst aneuploidy rates across female age. Fertil Steril. 2021;116(5):1320–7.
Su MT, Tsai PY, Tsai HL, Chen YC, Kuo PL. miR-346 and miR-582-3p-regulated EG-VEGF expression and trophoblast invasion via matrix metalloproteinases 2 and 9. BioFactors. 2017;43(2):210–9.
Liu M, Wang Y, Lu H, Wang H, Shi X, Shao X, et al. miR-518b enhances human trophoblast cell proliferation through targeting Rap1b and activating Ras-MAPK signal. Front Endocrinol. 2018;9:100.
Cai J-L, Liu L-L, Hu Y, Jiang X-M, Qiu H-L, Sha A-G, et al. Polychlorinated biphenyls impair endometrial receptivity in vitro via regulating mir-30d expression and epithelial mesenchymal transition. Toxicology. 2016;365:25–34.
Shi S, Tan Q, Liang J, Cao D, Wang S, Liang J, et al. Placental trophoblast cell-derived exosomal microRNA-1290 promotes the interaction between endometrium and embryo by targeting LHX6. Mol Ther-Nucleic Acids. 2021;26:760–72.
Kang Y-J, Lees M, Matthews LC, Kimber SJ, Forbes K, Aplin JD. miR-145 suppresses embryo–epithelial juxtacrine communication at implantation by modulating maternal IGF1R. J Cell Sci. 2015;128(4):804–14.
Liu X, Zhao H, Li W, Bao H, Qu Q, Ma D. Up-regulation of miR-145 may contribute to repeated implantation failure after IVF–embryo transfer by targeting PAI-1. Reprod Biomed Online. 2020;40(5):627–36.
Huang K, Chen G, Fan W, Hu L. miR-23a-3p increases endometrial receptivity via CUL3 during embryo implantation. J Mol Endocrinol. 2020;65(2):35–44.
Akbar R, Ullah K, Rahman TU, Cheng Y, Pang H-Y, Jin L-Y, et al. miR-183-5p regulates uterine receptivity and enhances embryo implantation. J Mol Endocrinol. 2020;64(1):43–52.
Li L, Gou J, Yi T, Li Z. MicroRNA-30a-3p regulates epithelial-mesenchymal transition to affect embryo implantation by targeting Snai2. Biol Reprod. 2019;100(5):1171–9.
Liang J, Cao D, Zhang X, Liu L, Tan Q, Shi S, et al. miR-192-5p suppresses uterine receptivity formation through impeding epithelial transformation during embryo implantation. Theriogenology. 2020;157:360–71.
Zhou T, Ni T, Li Y, Zhang Q, Yan J, Chen ZJ. circFAM120A participates in repeated implantation failure by regulating decidualization via the miR-29/ABHD5 axis. FASEB J. 2021;35(9): e21872.
Shi L, Zhu L, Gu Q, Kong C, Liu X, Zhu Z. LncRNA MALAT1 promotes decidualization of endometrial stromal cells via sponging miR-498-3p and targeting histone deacetylase 4. Cell Biol Int. 2022;46(8):1264–74.
Zhang Q, Zhang H, Jiang Y, Xue B, Diao Z, Ding L, et al. MicroRNA-181a is involved in the regulation of human endometrial stromal cell decidualization by inhibiting Krüppel-like factor 12. Reprod Biol Endocrinol. 2015;13(1):1–9.
Tan Q, Shi S, Liang J, Cao D, Wang S, Wang Z. Endometrial cell-derived small extracellular vesicle miR-100-5p promotes functions of trophoblast during embryo implantation. Mol Ther-Nucleic Acids. 2021;23:217–31.
Liu Y, Mei Q, Shen Q, Yang J, Zou M, Li J, et al. miR-320a-3p levels in human granulosa cells: a promising bio-marker of good quality embryo and clinical pregnancy after IVF/ICSI. 2021;20(1):160.
Ying W, Riopel M, Bandyopadhyay G, Dong Y, Birmingham A, Seo JB, et al. Adipose tissue macrophage-derived exosomal miRNAs can modulate in vivo and in vitro insulin sensitivity. Cell. 2017;171(2):372–84. e12.
Kuokkanen S, Chen B, Ojalvo L, Benard L, Santoro N, Pollard JW. Genomic profiling of microRNAs and messenger RNAs reveals hormonal regulation in microRNA expression in human endometrium. Biol Reprod. 2010;82(4):791–801.
Altmäe S, Koel M, Võsa U, Adler P, Suhorutšenko M, Laisk-Podar T, et al. Meta-signature of human endometrial receptivity: a meta-analysis and validation study of transcriptomic biomarkers. Sci Rep. 2017;7(1):1–15.
Altmäe S, Esteban FJ, Stavreus-Evers A, Simón C, Giudice L, Lessey BA, et al. Guidelines for the design, analysis and interpretation of ‘omics’ data: focus on human endometrium. Hum Reprod Update. 2014;20(1):12–28.
Kurita T, Medina R, Schabel AB, Young P, Gama P, Parekh TV, et al. The activation function-1 domain of estrogen receptor α in uterine stromal cells is required for mouse but not human uterine epithelial response to estrogen. Differentiation. 2005;73(6):313–22.
Martı́n J, Domı́nguez F, Ávila S, Castrillo JL, Remohı́ J, Pellicer A, et al. Human endometrial receptivity: gene regulation. J Reprod Immunol. 2002;55(1–2):131–9.
Loke H, Rainczuk K, Dimitriadis E. MicroRNA biogenesis machinery is dysregulated in the endometrium of infertile women suggesting a role in receptivity and infertility. J Histochem Cytochem. 2019;67(8):589–99.
Stahl PD, Raposo G. Extracellular vesicles: exosomes and microvesicles, integrators of homeostasis. Physiology. 2019;34(3):169–77.
Kresowik JD, Devor EJ, Van Voorhis BJ, Leslie KK. MicroRNA-31 is significantly elevated in both human endometrium and serum during the window of implantation: a potential biomarker for optimum receptivity. Biol Reprod. 2014;91(1):17, 1–6.
Altmäe S, Martinez-Conejero JA, Esteban FJ, Ruiz-Alonso M, Stavreus-Evers A, Horcajadas JA, et al. MicroRNAs miR-30b, miR-30d, and miR-494 regulate human endometrial receptivity. Reprod Sci. 2013;20(3):308–17.
Haraguchi H, Saito-Fujita T, Hirota Y, Egashira M, Matsumoto L, Matsuo M, et al. MicroRNA-200a locally attenuates progesterone signaling in the cervix, preventing embryo implantation. Mol Endocrinol. 2014;28(7):1108–17.
Riyanti A, Febri RR, Zakirah SC, Harzif AK, Rajuddin R, Muharam R, et al. Suppressing HOXA-10 gene expression by MicroRNA 135b during the window of implantation in infertile women. J Reprod Infertil. 2020;21(3):217.
Li R, Qiao J, Wang L, Li L, Zhen X, Liu P, et al. MicroRNA array and microarray evaluation of endometrial receptivity in patients with high serum progesterone levels on the day of hCG administration. Reprod Biol Endocrinol. 2011;9(1):1–9.
Luo X, Yang R, Bai Y, Li L, Lin N, Sun L, et al. Binding of microRNA-135a (miR-135a) to homeobox protein A10 (HOXA10) mRNA in a high-progesterone environment modulates the embryonic implantation factors beta3-integrin (ITGβ3) and empty spiracles homeobox-2 (EMX2). Ann Transl Med. 2021;9(8):662.
Wang Q, Ai H, Li X, Tian H, Ning B, Zhang M, et al. Association of miRNA-145 with the occurrence and prognosis of hydrosalpinx-induced defective endometrial receptivity. Bosn J Basic Med Sci. 2021;21(1):81.
Wei P, Wang H, Li Y, Guo R. Nucleolar small molecule RNA SNORA75 promotes endometrial receptivity by regulating the function of miR-146a-3p and ZNF23. Aging (Albany NY). 2021;13(11):14924.
Wang Y, Lv Y, Gao S, Zhang Y, Sun J, Gong C, et al. MicroRNA profiles in spontaneous decidualized menstrual endometrium and early pregnancy decidua with successfully implanted embryos. PLoS ONE. 2016;11(1): e0143116.
Estella C, Herrer I, Moreno-Moya JM, Quiñonero A, Martínez S, Pellicer A, et al. miRNA signature and Dicer requirement during human endometrial stromal decidualization in vitro. PLoS ONE. 2012;7(7): e41080.
Tochigi H, Kajihara T, Mizuno Y, Mizuno Y, Tamaru S, Kamei Y, et al. Loss of miR-542-3p enhances IGFBP-1 expression in decidualizing human endometrial stromal cells. Sci Rep. 2017;7(1):1–10.
Vilella F, Moreno-Moya JM, Balaguer N, Grasso A, Herrero M, Martínez S, et al. Hsa-miR-30d, secreted by the human endometrium, is taken up by the pre-implantation embryo and might modify its transcriptome. Development. 2015;142(18):3210–21.
Zheng Q, Zhang D, u Yang Y, Cui X, Sun J, Liang C, et al. MicroRNA-200c impairs uterine receptivity formation by targeting FUT4 and α 1, 3-fucosylation. Cell Death Differ. 2017;24(12):2161–72.
Freis A, Keller A, Ludwig N, Meese E, Jauckus J, Rehnitz J, et al. Altered miRNA-profile dependent on ART outcome in early pregnancy targets Wnt-pathway. Reproduction. 2017;154(6):799–805.
Yang Q, Gu W-W, Gu Y, Yan N-N, Mao Y-Y, Zhen X-X, et al. Association of the peripheral blood levels of circulating microRNAs with both recurrent miscarriage and the outcomes of embryo transfer in an in vitro fertilization process. J Transl Med. 2018;16(1):1–14.
Azhari F, Pence S, Hosseini MK, Balci BK, Cevik N, Bastu E, et al. The role of the serum exosomal and endometrial microRNAs in recurrent implantation failure. J Matern-Fetal Neonatal Med. 2022;35(5):815–25.
Mo B, Vendrov AE, Palomino WA, DuPont BR, Apparao K, Lessey BA. ECC-1 cells: a well-differentiated steroid-responsive endometrial cell line with characteristics of luminal epithelium. Biol Reprod. 2006;75(3):387–94.
Moreno-Moya JM, Vilella F, Martínez S, Pellicer A, Simón C. The transcriptomic and proteomic effects of ectopic overexpression of miR-30d in human endometrial epithelial cells. Mol Hum Reprod. 2014;20(6):550–66.
Ng YH, Rome S, Jalabert A, Forterre A, Singh H, Hincks CL, et al. Endometrial exosomes/microvesicles in the uterine microenvironment: a new paradigm for embryo-endometrial cross talk at implantation. PLoS ONE. 2013;8(3): e58502.
Balaguer N, Moreno I, Herrero M, González M, Simón C, Vilella F. Heterogeneous nuclear ribonucleoprotein C1 may control miR-30d levels in endometrial exosomes affecting early embryo implantation. MHR: Basic Sci Reprod Med. 2018;24(8):411–25.
Coughlan C, Ledger W, Wang Q, Liu F, Demirol A, Gurgan T, et al. Recurrent implantation failure: definition and management. Reprod Biomed Online. 2014;28(1):14–38.
Bashiri A, Halper KI, Orvieto R. Recurrent implantation failure-update overview on etiology, diagnosis, treatment and future directions. Reprod Biol Endocrinol. 2018;16(1):1–18.
Revel A, Achache H, Stevens J, Smith Y, Reich R. MicroRNAs are associated with human embryo implantation defects. Hum Reprod. 2011;26(10):2830–40.
Shi C, Shen H, Fan L-J, Guan J, Zheng X-B, Chen X, et al. Endometrial microRNA signature during the window of implantation changed in patients with repeated implantation failure. Chin Med J. 2017;130(5):566.
Azarpoor A, Ardeshirylajimi A, Mohammadi YS, Dehghan Z, Salehi M. The expression of miR-31 and its target gene FOXP3 in recurrent implantation failure patients. 2020;8(4):389–95.
Zhang Q, Ni T, Dang Y, Ding L, Jiang J, Li J, et al. MiR-148a-3p may contribute to flawed decidualization in recurrent implantation failure by modulating HOXC8. J Assist Reprod Genet. 2020;37(10):2535–44.
Chen CH, Lu F, Yang WJ, Yang PE, Chen WM, Kang ST, et al. A novel platform for discovery of differentially expressed microRNAs in patients with repeated implantation failure. Fertil Steril. 2021;116(1):181–88.
Zhao F, Guo Y, Shi Z, Wu M, Lv Y, Song W. hsa_circ_001946 elevates HOXA10 expression and promotes the development of endometrial receptivity via sponging miR-135b. Diagn Pathol. 2021;16(1):1–10.
Zhao Y, He D, Zeng H, Luo J, Yang S, Chen J, et al. Expression and significance of miR-30d-5p and SOCS1 in patients with recurrent implantation failure during implantation window. Reprod Biol Endocrinol. 2021;19(1):1–10.
Mei F, Kong C, Wang Y, Zhuang J, Xue P, Qi H, et al. MiR-133b Improves decidualization of endometrial stromal cells by targeting KLF12 in recurrent implantation failure. 2021. https://doi.org/10.21203/rs.3.rs-771502/v1
Liu C, Wang M, Zhang H, Sui C. Altered microRNA profiles of extracellular vesicles secreted by endometrial cells from women with recurrent implantation failure. Reprod Sci. 2021;28(7):1945–55.
Terrinoni A, Calabrese C, Basso D, Aita A, Caporali S, Plebani M, et al. The circulating miRNAs as diagnostic and prognostic markers. Clin Chem Lab Med (CCLM). 2019;57(7):932–53.
Author information
Authors and Affiliations
Contributions
Idea development: Elham Azizi and Mohammad Naji; literature search and data analysis: Elham Azizi and Zahra Shams Mofarahe; manuscript drafting: Elham Azizi and Zahra Shams Mofarahe; critical revision: Mohammad Naji.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Azizi, E., Mofarahe, Z.S. & Naji, M. MicroRNAs, small regulatory elements with significant effects on human implantation: a review. J Assist Reprod Genet 40, 697–717 (2023). https://doi.org/10.1007/s10815-023-02735-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-023-02735-w