Abstract
Objective
Given that the molecular diagnosis of autosomal dominant polycystic kidney disease (ADPKD) is complicated, we aim to apply blocker displacement amplification (BDA) on the mutational screening of PKD1 and PKD2.
Methods
A total of 35 unrelated families with ADPKD were recruited from the Center for Reproductive Medicine, Women and Children’s Hospital of Chongqing Medical University (Chongqing, China), from October 2018 to October 2021. Long-range PCR followed by next-generation sequencing were applied for resequencing of PKD1 and PKD2, and the putatively disease-causative variants were verified with BDA. The effects of ADPKD on male and female infertility and the factors influencing the clinical outcomes of preimplantation genetic testing (PGT) for ADPKD were investigated.
Results
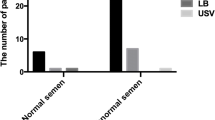
A total of 26 PKD1 variants and 5 PKD2 variants were identified, of which 13 were newly discovered. The BDA system worked effectively for eliminating the interference of pseudogenes in genetic testing of PKD1 (1–33 exons) with different concentrations of genome DNA. The females with ADPKD have no specific infertility factors, while 68.2% of the affected men were with abnormal sperm concentration and/or motility with an indefinite genotype–phenotype relationship. As for PGT, the fertilization rate of couples with the male partner having ADPKD was relatively lower compared to those with the female partner being affected. The ADPKD patients receiving PGT usually achieved high rates of live births.
Conclusion
These findings expanded the variant spectrum of PKD genes and emphasized the application prospect of blocker displacement amplification on PKD1-related genetic diagnosis.


Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in the article and the supplementary information files.
References
Cornec-Le Gall E, Alam A, Perrone RD. Autosomal dominant polycystic kidney disease. Lancet. 2019;393(10174):919–35.
Saini AK, Saini R, Singh S. Autosomal dominant polycystic kidney disease and pioglitazone for its therapy: a comprehensive review with an emphasis on the molecular pathogenesis and pharmacological aspects. Mol Med. 2020;26(1):128.
Lanktree MB, et al. Prevalence estimates of polycystic kidney and liver disease by population sequencing. J Am Soc Nephrol. 2018;29(10):2593–600.
Willey CJ, et al. Prevalence of autosomal dominant polycystic kidney disease in the European Union. Nephrol Dial Transplant. 2017;32(8):1356–63.
Nowak KL, Hopp K. Metabolic reprogramming in autosomal dominant polycystic kidney disease: evidence and therapeutic potential. Clin J Am Soc Nephrol. 2020;15(4):577–84.
Raj S, Singh RG, Das P. Mutational screening of PKD1 and PKD2 in Indian ADPKD patients identified 95 genetic variants. Mutat Res. 2020;821:111718.
He WB, et al. Novel mutations of PKD genes in Chinese patients suffering from autosomal dominant polycystic kidney disease and seeking assisted reproduction. BMC Med Genet. 2018;19(1):186.
Porath B, et al. Mutations in GANAB, encoding the glucosidase iialpha subunit, cause autosomal-dominant polycystic kidney and liver disease. Am J Hum Genet. 2016;98(6):1193–207.
Huynh VT, et al. Clinical spectrum, prognosis and estimated prevalence of DNAJB11-kidney disease. Kidney Int. 2020;98(2):476–87.
Cornec-Le Gall E, Torres VE, Harris PC. Genetic complexity of autosomal dominant polycystic kidney and liver diseases. J Am Soc Nephrol. 2018;29(1):13–23.
Lanktree MB, et al. Insights into autosomal dominant polycystic kidney disease from genetic studies. Clin J Am Soc Nephrol. 2020;16:790.
Hwang YH, et al. Refining genotype-phenotype correlation in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2016;27(6):1861–8.
Cornec-Le Gall E, et al. The PROPKD score: a new algorithm to predict renal survival in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2016;27(3):942–51.
Cornec-Le Gall E, et al. Type of PKD1 mutation influences renal outcome in ADPKD. J Am Soc Nephrol. 2013;24(6):1006–13.
Chebib FT, Torres VE. Autosomal dominant polycystic kidney disease: core curriculum 2016. Am J Kidney Dis. 2016;67(5):792–810.
Shi H, et al. A novel monogenic preimplantation genetic testing strategy for sporadic polycystic kidney caused by de novo PKD1 mutation. Clin Genet. 2021;99(2):250–8.
Cornec-Le Gall E, et al. Genetics and pathogenesis of autosomal dominant polycystic kidney disease: 20 years on. Hum Mutat. 2014;35(12):1393–406.
Li W, et al. The mutation-free embryo for in vitro fertilization selected by MALBAC-PGD resulted in a healthy live birth from a family carrying PKD 1 mutation. J Assist Reprod Genet. 2017;34(12):1653–8.
Bullich G, et al. A kidney-disease gene panel allows a comprehensive genetic diagnosis of cystic and glomerular inherited kidney diseases. Kidney Int. 2018;94(2):363–71.
De Rycke M, Berckmoes V. Preimplantation genetic testing for monogenic disorders. Genes (Basel). 2020;11(8).
Berckmoes V, et al. Factors influencing the clinical outcome of preimplantation genetic testing for polycystic kidney disease. Hum Reprod. 2019;34(5):949–58.
Wu LR, et al. Multiplexed enrichment of rare DNA variants via sequence-selective and temperature-robust amplification. Nat Biomed Eng. 2017;1:714–23.
Pei Y, et al. Imaging-based diagnosis of autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2015;26(3):746–53.
Pei Y, et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol. 2009;20(1):205–12.
Kopanos C, et al. VarSome: the human genomic variant search engine. Bioinformatics. 2019;35(11):1978–80.
Kataoka H, et al. Prediction of renal prognosis in patients with autosomal dominant polycystic kidney disease using PKD1/PKD2 mutations. J Clin Med. 2020;9(1).
Li J, et al. VarCards: an integrated genetic and clinical database for coding variants in the human genome. Nucleic Acids Res. 2018;46(D1):D1039–48.
Richards S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–24.
Baldi E, et al. Extended semen examinations in the sixth edition of the WHO Laboratory Manual for the Examination and Processing of Human Semen: contributing to the understanding of the function of the male reproductive system. Fertil Steril. 2022;117(2):252–7.
Organization, GWH, WHO laboratory manual for the examination and processing of human semen, sixth edition. License: CC BY-NC-SA 3.0 IGO., 2021.
Tu C, et al. Genetic underpinnings of asthenozoospermia. Best Pract Res Clin Endocrinol Metab. 2020;34(6):101472.
Huang L, et al. Single-cell whole-genome amplification and sequencing: methodology and applications. Annu Rev Genomics Hum Genet. 2015;16:79–102.
Nie X, Arend LJ. Pkd1 is required for male reproductive tract development. Mech Dev. 2013;130(11-12):567–76.
Nie X, Arend LJ. Novel roles of Pkd2 in male reproductive system development. Differentiation. 2014;87(3-4):161–71.
Su Q, et al. Structure of the human PKD1-PKD2 complex. Science. 2018;361(6406).
The European Polycystic Kidney Disease Consortium. The polycystic kidney disease 1 gene encodes a 14 kb transcript and lies within a duplicated region on chromosome 16. Cell. 1994;77(6):881–94.
Kim JA, Blumenfeld JD, Prince MR. Seminal vesicles in autosomal dominant polycystic kidney disease. In: Li X, editor. Polycystic kidney disease. Brisbane (AU); 2015.
Houston BJ, et al. A systematic review of the validated monogenic causes of human male infertility: 2020 update and a discussion of emerging gene-disease relationships. Hum Reprod Update. 2021;28(1):15–29.
Funding
This study was supported by grants from the Natural Science Foundation of Chongqing (CSTC2021JCYJ-MSXMX0722) and the Chongqing Health Center for Women and Children (2020YJMS01).
Author information
Authors and Affiliations
Contributions
T.T Lin, J.F Luo, D.Y Liu, and G.N Huang conceived and designed the study. B.H Dong, W Zhang, and K Chen carried out the experiments. H.B Yu and Y.Z Xiang provided the clinical samples. D.Y Liu conducted the PGT cycle. T.T Lin wrote the manuscript. D.Y Liu and G.N Huang critically commented on and edited the manuscript. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics statement
The studies involving human participants were reviewed and approved by the Clinical Application and Ethics Committee of Human Assisted Reproductive Technology of Chongqing Health Center for Women and Children.
Consent
Informed consent was obtained from the proband/participants.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, T., Luo, J., Yu, H. et al. Blocker displacement amplification-based genetic diagnosis for autosomal dominant polycystic kidney disease and the clinical outcomes of preimplantation genetic testing. J Assist Reprod Genet 40, 783–792 (2023). https://doi.org/10.1007/s10815-023-02722-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-023-02722-1