Abstract
Purpose
To investigate the genetic causes of polyspermy and total fertilization failure (TFF) in two independent male patients suffering from male infertility.
Methods
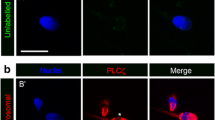
Immunofluorescence (IF) staining was used to detect the localization of the PLCζ protein in sperm and the maternal pronucleus in the zygote. Genomic DNA samples were extracted from the peripheral blood of patients and their families. The ExAC database was used to identify the frequency of corresponding mutations. The PLCZ1 mutations were validated by Sanger sequencing. The pathogenicity of the identified mutations and their possible effects on the protein were assessed using in silico tools and molecular modeling.
Results
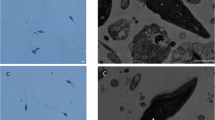
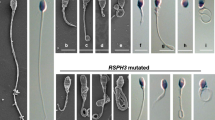
We identified a reported homozygous mutation c.588C > A (p.Cys196Ter) and a compound heterozygous mutation c.2 T > C(p.Met1Thr)/c.590G > A (p.Arg197His) with one novel mutation in PLCZ1. The IF results showed that these multipronuclear zygotes formed as a result of polyspermy. In silico analysis predicted that the mutations result in disease-causing proteins. IF staining revealed that PLCζ is abnormally localized in the sperm samples from the two affected patients. Assisted oocyte activation (AOA) successfully rescued polyspermy and TFF and achieved pregnancy in two patients with the PLCZ1 mutation.
Conclusion
We identified a homozygous mutation in PLCZ1 (c.588C > A [p.Cys196Ter]) in a male patient with polyspermy after in vitro fertilization (IVF) as well as a compound heterozygous mutation c.2 T > C(p.Met1Thr)/c.590G > A (p.Arg197His) with one novel mutation in a male patient with fertilization failure after intracytoplasmic sperm injection (ICSI), and we provide evidence that the homozygous mutation can cause polyspermy and the compound heterozygous mutation can cause fertilization failure.





Similar content being viewed by others
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Clift D, Schuh M. Restarting life: fertilization and the transition from meiosis to mitosis. Nat Rev Mol Cell Biol. 2013;14(9):549–62.
Rosenbusch BE. A preliminary concept, deduced from cytogenetic analyses, for explaining different types of multipronuclear oocytes obtained after intracytoplasmic sperm injection. Fertil Steril. 2010;94(6):2479–81.
Kai Y, et al. Diagnosis of abnormal human fertilization status based on pronuclear origin and/or centrosome number. J Assist Reprod Genet. 2015;32(11):1589–95.
Wozniak KL, Carlson AE. Ion channels and signaling pathways used in the fast polyspermy block. Mol Reprod Dev. 2020;87(3):350–7.
Evans JP. Preventing polyspermy in mammalian eggs-Contributions of the membrane block and other mechanisms. Mol Reprod Dev. 2020;87(3):341–9.
Egozcue S, et al. Diploid sperm and the origin of triploidy. Hum Reprod. 2002;17(1):5–7.
Rosenbusch B, Schneider M, Sterzik K. The chromosomal constitution of multipronuclear zygotes resulting from in-vitro fertilization. Hum Reprod. 1997;12(10):2257–62.
Gardner AJ, Evans JP. Mammalian membrane block to polyspermy: new insights into how mammalian eggs prevent fertilisation by multiple sperm. Reprod Fertil Dev. 2006;18(1–2):53–61.
Dai J, et al. Homozygous pathogenic variants in ACTL9 cause fertilization failure and male infertility in humans and mice. Am J Hum Genet. 2021;108(3):469–81.
Dai J, et al. Novel homozygous variations in PLCZ1 lead to poor or failed fertilization characterized by abnormal localization patterns of PLCzeta in sperm. Clin Genet. 2020;97(2):347–51.
Wang J, et al. Novel bi-allelic variants in ACTL7A are associated with male infertility and total fertilization failure. Hum Reprod. 2021;36(12):3161–9.
Mu J, et al. The identification of novel mutations in PLCZ1 responsible for human fertilization failure and a therapeutic intervention by artificial oocyte activation. Mol Hum Reprod. 2020;26(2):80–7.
Yan Z, et al. Novel mutations in PLCZ1 cause male infertility due to fertilization failure or poor fertilization. Hum Reprod. 2020;35(2):472–81.
Yuan P, et al. A novel homozygous mutation of phospholipase C zeta leading to defective human oocyte activation and fertilization failure. Hum Reprod. 2020;35(4):977–85.
Nozawa K, et al. Sperm-borne phospholipase C zeta-1 ensures monospermic fertilization in mice. Sci Rep. 2018;8(1):1315.
Yeste M, et al. Oocyte activation deficiency: a role for an oocyte contribution? Hum Reprod Update. 2016;22(1):23–47.
Iwao Y. Egg activation in physiological polyspermy. Reproduction. 2012;144(1):11–22.
Yoon SY, et al. Human sperm devoid of PLC, zeta 1 fail to induce Ca(2+) release and are unable to initiate the first step of embryo development. J Clin Invest. 2008;118(11):3671–81.
Ramadan WM, et al. Oocyte activation and phospholipase C zeta (PLCzeta): diagnostic and therapeutic implications for assisted reproductive technology. Cell Commun Signal. 2012;10(1):12.
Saunders CM, et al. PLC zeta: a sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development. 2002;129(15):3533–44.
Shen J, et al. Successful PGD for late infantile neuronal ceroid lipofuscinosis achieved by combined chromosome and TPP1 gene analysis. Reprod Biomed Online. 2013;27(2):176–83.
Yoon HJ, et al. Analysis of clinical outcomes with respect to spermatozoan origin after artificial oocyte activation with a calcium ionophore. J Assist Reprod Genet. 2013;30(12):1569–75.
McKenna A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20(9):1297–303.
Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38(16): e164.
Peng Y, et al. Nucleoporin37 may play a role in early embryo development in human and mice. Mol Hum Reprod. 2022;28(6):gaac017. https://doi.org/10.1093/molehr/gaac017.
Kelley LA, et al. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc. 2015;10(6):845–58.
Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J Mol Graph. 1996;14(1):33–8.
van de Werken C, et al. Paternal heterochromatin formation in human embryos is H3K9/HP1 directed and primed by sperm-derived histone modifications. Nat Commun. 2014;5:5868.
Hachem A, et al. PLCζ is the physiological trigger of the Ca2+ oscillations that induce embryogenesis in mammals but conception can occur in its absence. Development. 2017;144(16):2914–24.
Bhakta HH, Refai FH, Avella MA. The molecular mechanisms mediating mammalian fertilization. Development. 2019;146(15):dev176966.
Stewart-Savage J, Bavister BD. A cell surface block to polyspermy occurs in golden hamster eggs. Dev Biol. 1988;128(1):150–7.
Que EL, et al. Zinc sparks induce physiochemical changes in the egg zona pellucida that prevent polyspermy. Integr Biol (Camb). 2017;9(2):135–44.
Xiong B, et al. A unique egg cortical granule localization motif is required for ovastacin sequestration to prevent premature ZP2 cleavage and ensure female fertility in mice. PLoS Genet. 2017;13(1): e1006580.
Sato K. Polyspermy-preventing mechanisms in mouse eggs fertilized in vitro. J Exp Zool. 1979;210(2):353–9.
Kouchi Z, et al. The role of EF-hand domains and C2 domain in regulation of enzymatic activity of phospholipase Czeta. J Biol Chem. 2005;280(22):21015–21.
Torra-Massana M, et al. Novel phospholipase C zeta 1 mutations associated with fertilization failures after ICSI. Hum Reprod. 2019;34(8):1494–504.
Xin A, et al. Disruption in ACTL7A causes acrosomal ultrastructural defects in human and mouse sperm as a novel male factor inducing early embryonic arrest. Sci Adv. 2020;6(35):eaaz4796.
Nikiforaki D, et al. Effect of two assisted oocyte activation protocols used to overcome fertilization failure on the activation potential and calcium releasing pattern. Fertil Steril. 2016;105(3):798-806.e2.
Acknowledgements
We thank all families for participating in this study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peng, Y., Lin, Y., Deng, K. et al. Mutations in PLCZ1 induce male infertility associated with polyspermy and fertilization failure. J Assist Reprod Genet 40, 53–64 (2023). https://doi.org/10.1007/s10815-022-02670-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-022-02670-2