Abstract
Purpose
Can the risk factors that cause first trimester pregnancy loss in good-quality frozen-thawed embryo transfer (FET) cycles be predicted using machine learning algorithms?
Methods
This is a retrospective cohort study conducted at Sisli Memorial Hospital, ART and Reproductive Genetics Center, between January 2011 and May 2021. A total of 3805 good-quality FET cycles were included in the study. First trimester pregnancy loss rates were evaluated according to female age, paternal age, body mass index (BMI), diagnosis of infertility, endometrial preparation protocols (natural/artificial), embryo quality (top/good), presence of polycystic ovarian syndrome (PCOS), history of recurrent pregnancy loss (RPL), recurrent implantation failure (RIF), severe male infertility, adenomyosis and endometriosis.
Results
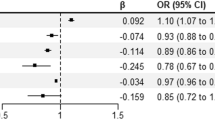
The first trimester pregnancy loss rate was 18.2% (693/ 3805). The presence of RPL increased first trimester pregnancy loss (OR = 7.729, 95%CI = 5.908–10.142, P = 0.000). BMI, which is > 30, increased first trimester pregnancy loss compared to < 25 (OR = 1.418, 95%CI = 1.025–1.950, P = 0.033). Endometrial preparation with artificial cycle increased first trimester pregnancy loss compared to natural cycle (OR = 2.101, 95%CI = 1.630–2.723, P = 0.000). Female age, which is 35–37, increased first trimester pregnancy loss compared to < 30 (OR = 1.617, 95%CI = 1.120–2.316, P = 0.018), and female age, which is > 37, increased first trimester pregnancy loss compared to < 30 (OR = 2.286, 95%CI = 1.146–4,38, P = 0.016). The presence of PCOS increased first trimester pregnancy loss (OR = 1.693, 95%CI = 1.198–2.390, P = 0.002). The number of previous IVF cycles, which is > 3, increased first trimester pregnancy loss compared to < 3 (OR = 2.182, 95%CI = 1.708–2.790, P = 0.000).
Conclusions
History of RPL, RIF, advanced female age, presence of PCOS, and high BMI (> 30 kg/m2) were the factors that increased first trimester pregnancy loss.


Similar content being viewed by others
References
Niederberger C, et al. Forty years of IVF. Fertility and Sterility. 2018;110(2):185-324 e5.
Cumming G, et al. The emotional burden of miscarriage for women and their partners: trajectories of anxiety and depression over 13 months BJOG: Int J Obstet Gynaecol. 2007;114(9):1138–45.
Jurkovic D, Overton C. , Bender-Atik R. Diagnosis and management of first trimester miscarriage. BMJ. 2013: 346
Quenby S, et al. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. The Lancet. 2021;397(10285):1658–1667.
Turner K, et al. Stress and anxiety scores in first and repeat IVF cycles: a pilot study. Plos One. 2013;8(5):e63743.
Winter E, et al. Early pregnancy loss following assisted reproductive technology treatment. Hum Reprod. 2002;17(12):3220–3.
Hipp H, et al. First trimester pregnancy loss after fresh and frozen in vitro fertilization cycles. Fertil Steril. 2016;105(3):722–8.
Uyar A, Bener A, Ciray HN. Predictive modeling of implantation outcome in an in vitro fertilization setting: an application of machine learning methods. Med Decis Making. 2015;35(6):714–25.
Yi Y, et al. A logistic model to predict early pregnancy loss following in vitro fertilization based on 2601 infertility patients. Reprod Biol Endocrinol. 2016;14(1):1–7.
Liu L, et al. Machine learning algorithms to predict early pregnancy loss after in vitro fertilization-embryo transfer with fetal heart rate as a strong predictor. Comput Methods Programs Biomed. 2020;196:105624.
Hassan MR, et al. A machine learning approach for prediction of pregnancy outcome following IVF treatment. Neural Comput Appl. 2020;32(7):2283–97.
Qiu J, et al. Personalized prediction of live birth prior to the first in vitro fertilization treatment: a machine learning method. J Transl Med. 2019;17(1):1–8.
Islam MN, et al. Machine learning to predict pregnancy outcomes: a systematic review, synthesizing framework and future research agenda. BMC Pregnancy Childbirth. 2022;22(1):1–19.
Practice Committee of the American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril. 2013; 99(1):63.
Mascarenhas M et al. Management of recurrent implantation failure: British Fertility Society policy and practice guideline. Human Fertil 2021:1–25.
Géron A. Hands-on machine learning with scikit-learn, Keras, and TensorFlow: concepts, tools, and techniques to build intelligent systems. 2019: " O'Reilly Media, Inc.".
Mitchell, T.M, Mitchell TM, Machine learning. Vol. 1. 1997: McGraw-hill New York.
Tan P-N, Steinbach M, Kumar V. Data mining introduction. Beijing: People’s Posts and Telecommunications Publishing House; 2006.
Breiman L. Random forests. Mach Learn. 2001;45(1):5–32.
Grekousis G, et al. Ranking the importance of demographic, socioeconomic, and underlying health factors on US COVID-19 deaths: a geographical random forest approach. Health Place. 2022;74: 102744.
Hosmer DW Jr, Lemeshow S, RX. Sturdivant, Applied logistic regression. Vol. 398. 2013: John Wiley & Sons.
Van Rossum G, Drake F. Python/C Api Manual-Python. 2009;3: CreateSpace.
Team RC. R: A language and environment for statistical computing. 2013
McKinney W. Data structures for statistical computing in python. in Proceedings of the 9th Python in Science Conference. 2010. Austin
Harris CR, et al. Array programming with NumPy. Nature. 2020;585(7825):357–62.
Hunter JD. Matplotlib: a 2D graphics environment. Compt Sci Eng. 2007;9(03):90–5.
Pedregosa F, et al. Scikit-learn: machine learning in Python. J Mach Learn Res. 2011;12:2825–30.
Team RC, R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Hosmer DW, Lemeshow S, Cook E. Applied logistic regression. 2nd ed. New York: Jhon Wiley and Sons Inc; 2000.
Carbonnel M, et al. Uterine factors in recurrent pregnancy losses. Fertil Steril. 2021;115(3):538–45.
Klimczak AM, et al. Role of the sperm, oocyte, and embryo in recurrent pregnancy loss. Fertil Steril. 2021;115(3):533–7.
Alecsandru D, et al. Immunologic causes and thrombophilia in recurrent pregnancy loss. Fertil Steril. 2021;115(3):561–6.
Morin SJ, et al. Translocations, inversions and other chromosome rearrangements. Fertil Steril. 2017;107(1):19–26.
Nguyen NMP, et al. Causative mutations and mechanism of androgenetic hydatidiform moles. Am J Human Genet. 2018;103(5):740–51.
Kuliev A, et al. Chromosomal abnormalities in a series of 6733 human oocytes in preimplantation diagnosis for age-related aneuploidies. Reprod Biomed Online. 2003;6(1):54–9.
Kim YJ, et al. Does a vitrified blastocyst stage embryo transfer program need hormonal priming for endometrial preparation? Journal of Obstetrics and Gynaecology Research. 2010;36(4):783–8.
Kawamura T, et al. Clinical outcomes of two different endometrial preparation methods for cryopreserved-thawed embryo transfer in patients with a normal menstrual cycle. Reprod Med Biol. 2007;6(1):53–7.
Alur-Gupta S, et al. Impact of method of endometrial preparation for frozen blastocyst transfer on pregnancy outcome: a retrospective cohort study. Fertil Steril. 2018;110(4):680–6.
Groenewoud ER, Cohlen BJ, Macklon NS. Programming the endometrium for deferred transfer of cryopreserved embryos: hormone replacement versus modified natural cycles. Fertil Steril. 2018;109(5):768–74.
Lathi RB, et al. Frozen blastocyst embryo transfer using a supplemented natural cycle protocol has a similar live birth rate compared to a programmed cycle protocol. J Assist Reprod Genet. 2015;32(7):1057–62.
Armas DFC et al. Frozen-thawed blastocyst transfer in natural cycle increase implantation rates compared artificial cycle. Gynecol Endocrinol. 2019.
Morozov V, et al. Natural cycle cryo-thaw transfer may improve pregnancy outcome. J Assist Reprod Genet. 2007;24(4):119–23.
Givens CR, et al. Outcomes of natural cycles versus programmed cycles for 1677 frozen–thawed embryo transfers. Reprod Biomed Online. 2009;19(3):380–4.
Tomás C, et al. Pregnancy loss after frozen-embryo transfer—a comparison of three protocols. Fertil Steril. 2012;98(5):1165–9.
Hancke K, et al. Patients undergoing frozen-thawed embryo transfer have similar live birth rates in spontaneous and artificial cycles. J Assist Reprod Genet. 2012;29(5):403–7.
Patel S, et al. Estradiol elicits proapoptotic and antiproliferative effects in human trophoblast cells. Biology of reproduction. 2015;93(3):7–1-10.
Teede HJ, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110(3):364–79.
Craig LB, Ke RW, Kutteh WH. Increased prevalence of insulin resistance in women with a history of recurrent pregnancy loss. Fertil Steril. 2002;78(3):487–90.
Tian L, et al. Insulin resistance increases the risk of spontaneous abortion after assisted reproduction technology treatment. J Clin Endocrinol Metab. 2007;92(4):1430–3.
Tesarik J. Effects of LH on oocyte yield and developmental competence. Hum Reprod. 2003;18(6):1358–60.
van der Spuy ZM, Dyer SJ. The pathogenesis of infertility and early pregnancy loss in polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2004;18(5):755–71.
Kurzawa R, et al. Comparison of embryological and clinical outcome in GnRH antagonist vs GnRH agonist protocols for in vitro fertilization in PCOS non-obese patients A prospective randomized study. J Assist Reprod Genet. 2008;25(8):365–74.
Tesarik J, Mendoza C. Nongenomic effects of 17 beta-estradiol on maturing human oocytes: relationship to oocyte developmental potential. J Clin Endocrinol Metab. 1995;80(4):1438–43.
Weghofer A, et al. Lack of association between polycystic ovary syndrome and embryonic aneuploidy. Fertil Steril. 2007;88(4):900–5.
Freis A, et al. Effects of a hyperandrogenaemic state on the proliferation and decidualization potential in human endometrial stromal cells. Arch Gynecol Obstet. 2017;295(4):1005–13.
Tuckerman EM, et al. Do androgens have a direct effect on endometrial function? An in vitro study. Fertil Steril. 2000;74(4):771–9.
Liu L, et al. A comparison of the miscarriage rate between women with and without polycystic ovarian syndrome undergoing IVF treatment. Eur J Obstet Gynecol Reprod Biol. 2014;176:178–82.
Shang K, et al. Endometrial abnormality in women with polycystic ovary syndrome. Reprod Sci. 2012;19(7):674–83.
Rittenberg V, et al. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online. 2011;23(4):421–39.
Sermondade N, et al. Female obesity is negatively associated with live birth rate following IVF: a systematic review and meta-analysis. Hum Reprod Update. 2019;25(4):439–51.
Bellver J, et al. Obesity and poor reproductive outcome: the potential role of the endometrium. Fertil Steril. 2007;88(2):446–51.
Gosman GG, Katcher HI, Legro RS. Obesity and the role of gut and adipose hormones in female reproduction. Hum Reprod Update. 2006;12(5):585–601.
Wang JX, Davies MJ, Norman RJ. Obesity increases the risk of spontaneous abortion during infertility treatment. Obes Res. 2002;10(6):551–4.
Yang X, Zheng B, Wang Y. Effect of pre-pregnancy body mass index on neonatal outcomes in women undergoing autologous frozen-thawed embryo transfer. Fertil Steril. 2021;4(116):1010–19.
Zhang J, et al. Effect of body mass index on pregnancy outcomes in a freeze-all policy: an analysis of 22,043 first autologous frozen-thawed embryo transfer cycles in China. BMC Med. 2019;17(1):1–9.
Bellver J, et al. Female obesity impairs in vitro fertilization outcome without affecting embryo quality. Fertil Steril. 2010;93(2):447–54.
Luke B, et al. Female obesity adversely affects assisted reproductive technology (ART) pregnancy and live birth rates†. Hum Reprod. 2010;26(1):245–52.
Bellver J et al. Obesity reduces uterine receptivity: clinical experience from 9,587 first cycles of ovum donation with normal weight donors. Fertil Steril. 2013;100(4):1050–1058. e2.
Comstock IA, et al. Does an increased body mass index affect endometrial gene expression patterns in infertile patients? A functional genomics analysis. Fertil Steril. 2017;107(3):740-748.e2.
Boots CE, Bernardi LA, Stephenson MD. Frequency of euploid miscarriage is increased in obese women with recurrent early pregnancy loss. Fertil Steril. 2014;102(2):455–9.
Tremellen K, Pearce K, Zander-Fox D. Increased miscarriage of euploid pregnancies in obese women undergoing cryopreserved embryo transfer. Reprod Biomed Online. 2017;34(1):90–7.
Schoolcraft WB, et al. Preimplantation aneuploidy testing for infertile patients of advanced maternal age: a randomized prospective trial. Fertil Steril. 2009;92(1):157–62.
Harton GL, et al. Diminished effect of maternal age on implantation after preimplantation genetic diagnosis with array comparative genomic hybridization. Fertil Steril. 2013;100(6):1695–703.
Seshadri S, et al. Assisted conception in women of advanced maternal age. Best Pract Res Clin Obstet Gynaecol. 2021;70:10–20.
Franasiak JM et al. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil steril. 2014;101(3):656–663. e1.
Ubaldi FM, et al. Advanced maternal age in IVF: still a challenge? The present and the future of its treatment. Front Endocrinol. 2019;10:94.
Van Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion. 2011;11(5):797–813.
Capalbo A, et al. Human female meiosis revised: new insights into the mechanisms of chromosome segregation and aneuploidies from advanced genomics and time-lapse imaging. Hum Reprod Update. 2017;23(6):706–22.
Hill MJ. Recurrent implantation failure: Sapere aude. Fertil Steril. 2021;116(6):1430–1.
Franasiak JM, et al. A review of the pathophysiology of recurrent implantation failure. Fertil Steril. 2021;116(6):1436–48.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ozer, G., Akca, A., Yuksel, B. et al. Prediction of risk factors for first trimester pregnancy loss in frozen-thawed good-quality embryo transfer cycles using machine learning algorithms. J Assist Reprod Genet 40, 279–288 (2023). https://doi.org/10.1007/s10815-022-02645-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-022-02645-3