Abstract
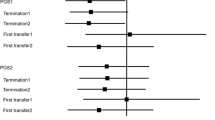
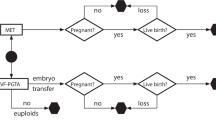
The hypothetical analysis presented offers insight into the effect of maternal age and protocol on the cost-effectiveness of PGT-A to reduce the risk of clinical miscarriage without materially jeopardising the chance of a first live birth when attempting to transfer every suitable embryo one at a time. Reflecting current practices, the diagnostic accuracy of PGT-A is sensitive to the prevalence of embryos with chromosome aneuploidy which increases with advancing maternal age, and the power of the test to discern a non-viable embryo is higher for older women and sensitive to protocol. PGT-A is effective to mitigate (reduce not eliminate) the risk of clinical miscarriage; however, excluding embryos with intermediate copy number results from transfer is detrimental to accomplishing a first live birth from a full cycle. Paradoxically, the number of blastocysts needed to marginalise the detriment is achieved only for some younger women (≤ 40 years) who are less likely to benefit by avoiding pregnancy loss; this also makes PGT-A an expensive adjuvant. The paradox can be avoided by excluding from transfer only embryos with a ‘uniform’ aneuploid test result, which mitigates the risk of miscarriage for all women with the potential to be cost-effective for those > 40 years.


Similar content being viewed by others
Data availability
Supplementary files are provided.
Code availability
Not applicable.
References
COGEN. A statement on the use of preimplantation genetic screening (PGS) of chromosomes for IVF patients 2015; https://ivf-worldwide.com/cogen/oep/publications/cogen-statement-on-the-use-of-preimplantation-genetic-screening-pgs-of-chromosomes-for-ivf-patients.html (last accessed 25/06/2022).
Mastenbroek S, de Wert G, Adashi EY. The imperative of responsible innovation in reproductive medicine. N Engl J Med. 2021;385:2096–100. https://doi.org/10.1056/nejmsb2101718.
Gleicher N, Barad DH, Patrizio P, Orvieto R. We have reached a dead end for preimplantation genetic testing for aneuploidy. Hum Reprod. 2022;deac052. Epub ahead of print. https://doi.org/10.1093/humrep/deac052
Cornelisse S, Zagers M, Kostova E, Fleischer K, van Wely M, Mastenbroek S. Preimplantation genetic testing for aneuploidies (abnormal number of chromosomes) in in vitro fertilisation. Cochrane Database Syst Rev. 2020;9:CD005291. https://doi.org/10.1002/14651858.cd005291.pub3.
Viotti M, Victor AR, Barnes FL, Zouves CG, Besser AG, Grifo JA, Cheng EH, Lee MS, Horcajadas JA, Corti L, Fiorentino F, Spinella F, Minasi MG, Greco E, Munné S. Using outcome data from one thousand mosaic embryo transfers to formulate an embryo ranking system for clinical use. Fertil Steril. 2021;115:1212–24. https://doi.org/10.1016/j.fertnstert.2020.11.041.
Capalbo A, Poli M, Rienzi L, Girardi L, Patassini C, Fabiani M, Cimadomo D, Benini F, Farcomeni A, Cuzzi J, Rubio C, Albani E, Sacchi L, Vaiarelli A, Figliuzzi M, Findikli N, Coban O, Boynukalin FK, Vogel I, Hoffmann E, Livi C, Levi-Setti PE, Ubaldi FM, Simón C. Mosaic human preimplantation embryos and their developmental potential in a prospective, non-selection clinical trial. Am J Hum Genet. 2021;108:2238–47. https://doi.org/10.1016/j.ajhg.2021.11.002.
Armstrong A, Miller J, Quinn M, Nguyen AV, Kwan L, Kroener L. To mask or not to mask mosaicism? The impact of reporting embryo mosaicism on reproductive potential. J Assist Reprod Genet. 2022;39:2035–42. https://doi.org/10.1007/s10815-022-02576-z.
Leigh D, Cram DS, Rechitsky S, Handyside A, Wells D, Munne S, Kahraman S, Grifo J, Katz-Jaffe M, Rubio C, Viotti M, Forman E, Xu K, Gordon T, Madjunkova S, Qiao J, Chen ZJ, Harton G, Gianaroli L, Simon C, Scott R, Simpson JL, Kuliev A. PGDIS position statement on the transfer of mosaic embryos 2021. Reprod Biomed Online. 2022;45:19–25. https://doi.org/10.1016/j.rbmo.2022.03.013.
Barad DH, Albertini DF, Molinari E, Gleicher N. IVF outcomes of embryos with abnormal PGT-A biopsy previously refused transfer: a prospective cohort study. Hum Reprod. 2022;37:1194–206. https://doi.org/10.1093/humrep/deac063.
Capalbo A, Cimadomo D, Rienzi L, Garcìa-Velasco JA, Simòn C, Ubaldi FM. Avoid mixing apples and oranges: blastocysts diagnosed with uniform whole chromosome aneuploidies are reproductively incompetent and their transfer is harmful. Hum Reprod. 2022;37:2213–4. https://doi.org/10.1093/humrep/deac149.
Ata B, Popovic M, Fatemi H. Correct assessment and interpretation of results determines the accuracy of any diagnostic test, and PGT-A is no exception. Hum Reprod. 2022;37:2214–6. https://doi.org/10.1093/humrep/deac150.
Barad DH, Albertini DF, Gleicher N. In science truth ultimately wins, and PGT-A is no exception. Hum Reprod. 2022;37:2216–8. https://doi.org/10.1093/humrep/deac151.
Tiegs AW, Tao X, Zhan Y, Whitehead C, Kim J, Hanson B, Osman E, Kim TJ, Patounakis G, Gutmann J, Castelbaum A, Seli E, Jalas C, Scott RT Jr. A multicenter, prospective, blinded, nonselection study evaluating the predictive value of an aneuploid diagnosis using a targeted next-generation sequencing-based preimplantation genetic testing for aneuploidy assay and impact of biopsy. Fertil Steril. 2021;115:627–37. https://doi.org/10.1016/j.fertnstert.2020.07.052.
Iwamoto A, Van Voorhis BJ, Summers KM, Sparks A, Mancuso AC. Intracytoplasmic sperm injection vs. conventional in vitro fertilization in patients with non-male factor infertility. Fertil Steril. 2022;118:465–72. https://doi.org/10.1016/j.fertnstert.2022.06.009.
Gillon R. Medical ethics: four principles plus attention to scope. BMJ. 1994;309:184–8. https://doi.org/10.1136/bmj.309.6948.184.
ESHRE PGT Consortium and SIG-Embryology Biopsy Working Group, Kokkali G, Coticchio G, Bronet F, Celebi C, Cimadomo D, Goossens V, Liss J, Nunes S, Sfontouris I, Vermeulen N, Zakharova E, De Rycke M. ESHRE PGT Consortium and SIG Embryology good practice recommendations for polar body and embryo biopsy for PGT. Hum Reprod Open. 2020;2020(3):hoaa020. https://doi.org/10.1093/hropen/hoaa020.
Scriven PN. Towards a better understanding of preimplantation genetic screening and cumulative reproductive outcome: transfer strategy, diagnostic accuracy and cost-effectiveness. AIMS Genetics. 2016;3:177–95. https://doi.org/10.3934/genet.2016.3.177.
Scriven PN. Towards a better understanding of preimplantation genetic screening for aneuploidy: insights from a virtual trial for women under the age of 40 when transferring embryos one at a time. Reprod Biol Endocrinol. 2017;15:49. https://doi.org/10.1186/s12958-017-0269-y.
Yan J, Qin Y, Zhao H, Sun Y, Gong F, Li R, Sun X, Ling X, Li H, Hao C, Tan J, Yang J, Zhu Y, Liu F, Chen D, Wei D, Lu J, Ni T, Zhou W, Wu K, Gao Y, Shi Y, Lu Y, Zhang T, Wu W, Ma X, Ma H, Fu J, Zhang J, Meng Q, Zhang H, Legro RS, Chen ZJ. Live birth with or without preimplantation genetic testing for aneuploidy. N Engl J Med. 2021;385:2047–58. https://doi.org/10.1056/nejmoa2103613.
Yang Z, Liu J, Collins GS, Salem SA, Liu X, Lyle SS, Peck AC, Sills ES, Salem RD. Selection of single blastocysts for fresh transfer via standard morphology assessment alone and with array CGH for good prognosis IVF patients: results from a randomized pilot study. Mol Cytogenet. 2012;5:24. https://doi.org/10.1186/1755-8166-5-24.
Author information
Authors and Affiliations
Contributions
The author is responsible for the content and writing of the article.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The author declares no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Scriven, P.N. Elucidating the PGT-A paradox: marginalising the detriment relegates the benefit. J Assist Reprod Genet 39, 2475–2481 (2022). https://doi.org/10.1007/s10815-022-02640-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-022-02640-8