Abstract
Purpose
To identify candidate variants in genes possibly associated with premature ovarian insufficiency (POI).
Methods
Fourteen women, from 7 families, affected by idiopathic POI were included. Additionally, 98 oocyte donors of the same ethnicity were enrolled as a control group. Whole-exome sequencing (WES) was performed in 14 women with POI to identify possibly pathogenic variants in genes potentially associated with the ovarian function. The candidate genes selected in POI patients were analysed within the exome results of oocyte donors.
Results
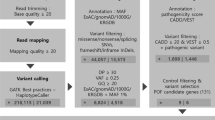
After the variant filtering in the WES analysis of 7 POI families, 23 possibly damaging genetic variants were identified in 22 genes related to POI or linked to ovarian physiology. All variants were heterozygous and five of the seven families carried two or more variants in different genes. We have described genes that have never been associated to POI pathology; however, they are involved in important biological processes for ovarian function. In the 98 oocyte donors of the control group, we found no potentially pathogenic variants among the 22 candidate genes.
Conclusion
WES has previously shown as an efficient tool to identify causative genes for ovarian failure. Although some studies have focused on it, and many genes are identified, this study proposes new candidate genes and variants, having potentially moderate/strong functional effects, associated with POI, and argues for a polygenic etiology of POI in some cases.


Similar content being viewed by others
References
Webber L, Davies M, Anderson R, Bartlett J, Braat D, Cartwright B, et al. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016;31(5):926–37. https://doi.org/10.1093/humrep/dew027.
Man L, Lekovich J, Rosenwaks Z, Gerhardt J. Fragile X-associated diminished ovarian reserve and primary ovarian insufficiency from molecular mechanisms to clinical manifestations. Front Mol Neurosci. 2017;10:290. https://doi.org/10.3389/fnmol.2017.00290.
Tenenbaum-Rakover Y, Weinberg-Shukron A, Renbaum P, Lobel O, Eideh H, Gulsuner S, et al. Minichromosome maintenance complex component 8 (MCM8) gene mutations result in primary gonadal failure. J Med Genet. 2015;52(6):391–9. https://doi.org/10.1136/jmedgenet-2014-102921.
Li L, Wang B, Zhang W, Chen B, Luo M, Wang J, et al. A homozygous NOBOX truncating variant causes defective transcriptional activation and leads to primary ovarian insufficiency. Hum Reprod. 2017;32(1):248–55. https://doi.org/10.1093/humrep/dew271.
Yang X, Zhang X, Jiao J, Zhang F, Pan Y, Wang Q, et al. Rare variants in FANCA induce premature ovarian insufficiency. Hum Genet. 2019;138(11–12):1227–36. https://doi.org/10.1007/s00439-019-02059-9.
Jaillard S, Bell K, Akloul L, Walton K, McElreavy K, Stocker WA, et al. New insights into the genetic basis of premature ovarian insufficiency: novel causative variants and candidate genes revealed by genomic sequencing. Maturitas. 2020;141:9–19. https://doi.org/10.1016/j.maturitas.2020.06.004.
Jiao X, Ke H, Qin Y, Chen ZJ. Molecular genetics of premature ovarian insufficiency. Trends Endocrinol Metab. 2018;29(11):795–807. https://doi.org/10.1016/j.tem.2018.07.002.
Tucker EJ, Grover SR, Bachelot A, Touraine P, Sinclair AH. Premature ovarian insufficiency: new perspectives on genetic cause and phenotypic spectrum. Endocr Rev. 2016;37(6):609–35. https://doi.org/10.1210/er.2016-1047.
Bouilly J, Beau I, Barraud S, Bernard V, Azibi K, Fagart J, et al. Identification of multiple gene mutations accounts for a new genetic architecture of primary ovarian insufficiency. J Clin Endocrinol Metab. 2016;101(12):4541–50. https://doi.org/10.1210/jc.2016-2152.
Patiño LC, Beau I, Carlosama C, Buitrago JC, González R, Suárez CF, et al. New mutations in non-syndromic primary ovarian insufficiency patients identified via whole-exome sequencing. Hum Reprod. 2017;32(7):1512–20. https://doi.org/10.1093/humrep/dex089.
Tang R, Yu Q. Novel variants in women with premature ovarian function decline identified via whole-exome sequencing. J Assist Reprod Genet. 2020;37(10):2487–502. https://doi.org/10.1007/s10815-020-01919-y.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–24. https://doi.org/10.1038/gim.2015.30.
Jaillard S, Akloul L, Beaumont M, Hamdi-Roze H, Dubourg C, Odent S, et al. Array-CGH diagnosis in ovarian failure: identification of new molecular actors for ovarian physiology. J Ovarian Res. 2016;9(1):63. https://doi.org/10.1186/s13048-016-0272-5.
Gorsi B, Hernandez E, Moore MB, Moriwaki M, Chow CY, Coelho E, et al. Causal and candidate gene variants in a large cohort of women with primary ovarian insufficiency. J Clin Endocrinol Metab. 2022;107(3):685–714. https://doi.org/10.1210/clinem/dgab775.
Turkyilmaz A, Alavanda C, Ates EA, Geckinli BB, Polat H, Gokcu M, Karakaya T, Cebi AH, Soylemez MA, Guney Aİ, Ata P, Arman A. Whole-exome sequencing reveals new potential genes and variants in patients with premature ovarian insufficiency. J Assist Reprod Genet. 2022;39(3):695–710. https://doi.org/10.1007/s10815-022-02408-0.
Lee AS, Rusch J, Lima AC, Usmani A, Huang N, Lepamets M, et al. Rare mutations in the complement regulatory gene CSMD1 are associated with male and female infertility. Nat Commun. 2019;10(1):4626. https://doi.org/10.1038/s41467-019-12522-w.
Flaws JA, Kugu K, Trbovich AM, DeSanti A, Tilly KI, Hirshfield AN, et al. Interleukin-1 beta-converting enzyme-related proteases (IRPs) and mammalian cell death: dissociation of IRP-induced oligonucleosomal endonuclease activity from morphological apoptosis in granulosa cells of the ovarian follicle. Endocrinology. 1995;136(11):5042–53. https://doi.org/10.1210/endo.136.11.7588240.
Bergeron L, Perez GI, Macdonald G, Shi L, Sun Y, Jurisicova A, et al. Defects in regulation of apoptosis in caspase-2-deficient mice. Genes Dev. 1998;12(9):1304–14. https://doi.org/10.1101/gad.12.9.1304.
Hanoux V, Pairault C, Bakalska M, Habert R, Livera G. Caspase-2 involvement during ionizing radiation-induced oocyte death in the mouse ovary. Cell Death Differ. 2007;14(4):671–81. https://doi.org/10.1038/sj.cdd.4402052.
Men NT, Kikuchi K, Furusawa T, Dang-Nguyen TQ, Nakai M, Fukuda A, et al. Expression of DNA repair genes in porcine oocytes before and after fertilization by ICSI using freeze-dried sperm. Anim Sci J. 2016;87(11):1325–33. https://doi.org/10.1111/asj.12554.
Sabatella M, Thijssen KL, Davó-Martínez C, Vermeulen W, Lans H. Tissue-specific DNA repair activity of ERCC-1/XPF-1. Cell Rep. 2021;34(2):108608. https://doi.org/10.1016/j.celrep.2020.108608.
Kawamura K, Kumagai J, Sudo S, Chun SY, Pisarska M, Morita H, et al. Paracrine regulation of mammalian oocyte maturation and male germ cell survival. Proc Natl Acad Sci U S A. 2004;101(19):7323–8. https://doi.org/10.1073/pnas.0307061101.
Hanna CB, Yao S, Patta MC, Jensen JT, Wu X. Expression of insulin-like 3 (INSL3) and differential splicing of its receptor in the ovary of rhesus macaques. Reprod Biol Endocrinol. 2010;8:150. https://doi.org/10.1186/1477-7827-8-150.
Satchell L, Glister C, Bleach EC, Glencross RG, Bicknell AB, Dai Y, et al. Ovarian expression of insulin-like peptide 3 (INSL3) and its receptor (RXFP2) during development of bovine antral follicles and corpora lutea and measurement of circulating INSL3 levels during synchronized estrous cycles. Endocrinology. 2013;154(5):1897–906. https://doi.org/10.1210/en.2012-2232.
Dickinson RE, Hryhorskyj L, Tremewan H, Hogg K, Thomson AA, McNeilly AS, et al. Involvement of the SLIT/ROBO pathway in follicle development in the fetal ovary. Reproduction. 2010;139(2):395–407. https://doi.org/10.1530/REP-09-0182.
Qin N, Fan XC, Zhang YY, Xu XX, Tyasi TL, Jing Y, et al. New insights into implication of the SLIT/ROBO pathway in the prehierarchical follicle development of hen ovary. Poult Sci. 2015;94(9):2235–46. https://doi.org/10.3382/ps/pev185.
Huntriss J, Hinkins M, Picton HM. cDNA cloning and expression of the human NOBOX gene in oocytes and ovarian follicles. Mol Hum Reprod. 2006;12(5):283–9. https://doi.org/10.1093/molehr/gal035.
Feranil JB, Isobe N, Nakao T. Immunolocalization of von Willebrand factor and vascular endothelial growth factor during follicular atresia in the swamp buffalo ovary. J Reprod Dev. 2005;51(4):419–26. https://doi.org/10.1262/jrd.17011.
Wang S, Zheng Y, Li J, Yu Y, Zhang W, Song M, et al. Single-cell transcriptomic atlas of primate ovarian aging. Cell. 2020;180(3):585-600.e19. https://doi.org/10.1016/j.cell.2020.01.009.
McRae RS, Johnston HM, Mihm M, O’Shaughnessy PJ. Changes in mouse granulosa cell gene expression during early luteinization. Endocrinology. 2005;146(1):309–17. https://doi.org/10.1210/en.2004-0999.
Li J, Li C, Li Q, Li WT, Li H, Li GX, et al. Identification of the Key microRNAs and miRNA-mRNA interaction networks during the ovarian development of hens. Animals (Basel). 2020;10(9). https://doi.org/10.3390/ani10091680.
Tan M, Tol HTAV, Rosenkranz D, Roovers EF, Damen MJ, Stout TAE, et al. PIWIL3 forms a complex with TDRKH in mammalian oocytes. Cells. 2020;9(6). https://doi.org/10.3390/cells9061356.
Kurowska P, Mlyczyńska E, Dawid M, Dupont J, Rak A. Role of vaspin in porcine ovary: effect on signaling pathways and steroid synthesis via GRP78 receptor and protein kinase A†. Biol Reprod. 2020;102(6):1290–305. https://doi.org/10.1093/biolre/ioaa027.
Johnson LE, DeLuca HF. Vitamin D receptor null mutant mice fed high levels of calcium are fertile. J Nutr. 2001;131(6):1787–91. https://doi.org/10.1093/jn/131.6.1787.
Panda DK, Miao D, Tremblay ML, Sirois J, Farookhi R, Hendy GN, et al. Targeted ablation of the 25-hydroxyvitamin D 1alpha -hydroxylase enzyme: evidence for skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci U S A. 2001;98(13):7498–503. https://doi.org/10.1073/pnas.131029498.
Talpur HS, Worku T, Rehman ZU, Dad R, Bhattarai D, Bano I, et al. Knockdown of melatonin receptor 1 and induction of follicle-stimulating hormone on the regulation of mouse granulosa cell function. Reprod Biol. 2017;17(4):380–8. https://doi.org/10.1016/j.repbio.2017.10.005.
Worku T, Wang K, Ayers D, Wu D, Ur Rehman Z, Zhou H, et al. Regulatory roles of ephrinA5 and its novel signaling pathway in mouse primary granulosa cell apoptosis and proliferation. Cell Cycle. 2018;17(7):892–902. https://doi.org/10.1080/15384101.2018.1456297.
Chen CL, Fu XF, Wang LQ, Wang JJ, Ma HG, Cheng SF, et al. Primordial follicle assembly was regulated by Notch signaling pathway in the mice. Mol Biol Rep. 2014;41(3):1891–9. https://doi.org/10.1007/s11033-014-3038-4.
Prasasya RD, Mayo KE. Notch signaling regulates differentiation and steroidogenesis in female mouse ovarian granulosa cells. Endocrinology. 2018;159(1):184–98. https://doi.org/10.1210/en.2017-00677.
Vanorny DA, Prasasya RD, Chalpe AJ, Kilen SM, Mayo KE. Notch signaling regulates ovarian follicle formation and coordinates follicular growth. Mol Endocrinol. 2014;28(4):499–511. https://doi.org/10.1210/me.2013-1288.
Hubbard N, Prasasya RD, Mayo KE. Activation of notch signaling by oocytes and Jag1 in mouse ovarian granulosa cells. Endocrinology. 2019;160(12):2863–76. https://doi.org/10.1210/en.2019-00564.
Li J, Zhao D, Guo C, Mi Y, Zhang C. Involvement of notch signaling in early chick ovarian follicle development. Cell Biol Int. 2016;40(1):65–73. https://doi.org/10.1002/cbin.10538.
Guo X, Wang Y, Chen Q, Yuan Z, Chen Y, Guo M, et al. The role of PTHLH in ovarian follicle selection, its transcriptional regulation and genetic effects on egg laying traits in hens. Front Genet. 2019;10:430. https://doi.org/10.3389/fgene.2019.00430.
Fowler PA, Anderson RA, Saunders PT, Kinnell H, Mason JI, Evans DB, et al. Development of steroid signaling pathways during primordial follicle formation in the human fetal ovary. J Clin Endocrinol Metab. 2011;96(6):1754–62. https://doi.org/10.1210/jc.2010-2618.
Richard S, Baltz JM. Preovulatory suppression of mouse oocyte cell volume-regulatory mechanisms is via signalling that is distinct from meiotic arrest. Sci Rep. 2017;7(1):702. https://doi.org/10.1038/s41598-017-00771-y.
Richard S, Tartia AP, Boison D, Baltz JM. Mouse oocytes acquire mechanisms that permit independent cell volume regulation at the end of oogenesis. J Cell Physiol. 2017;232(9):2436–46. https://doi.org/10.1002/jcp.25581.
Zhang LQ, Zhang XN, Gao Y, Ma XB, Dai LS, Jiang H, et al. Identification of differentially expressed proteins in the ovaries of menopausal women. Arch Gynecol Obstet. 2014;290(6):1179–86. https://doi.org/10.1007/s00404-014-3357-7.
Liu H, Wei X, Sha Y, Liu W, Gao H, Lin J, et al. Whole-exome sequencing in patients with premature ovarian insufficiency: early detection and early intervention. J Ovarian Res. 2020;13(1):114. https://doi.org/10.1186/s13048-020-00716-6.
Yang Y, Guo T, Liu R, Ke H, Xu W, Zhao S, et al. FANCL gene mutations in premature ovarian insufficiency. Hum Mutat. 2020;41(5):1033–41. https://doi.org/10.1002/humu.23997.
Guo T, Zheng Y, Li G, Zhao S, Ma J, Qin Y. Novel pathogenic mutations in minichromosome maintenance complex component 9 (MCM9) responsible for premature ovarian insufficiency. Fertil Steril. 2020;113(4):845–52. https://doi.org/10.1016/j.fertnstert.2019.11.015.
Daum H, Zlotogora J. Fanconi anemia gene variants in patients with gonadal dysfunction. Reprod Sci. 2022;29(5):1408–13. https://doi.org/10.1007/s43032-021-00582-7.
Chakravorty S, Hegde M. Inferring the effect of genomic variation in the new era of genomics. Hum Mutat. 2018;39(6):756–73. https://doi.org/10.1002/humu.23427.
Zhao M, Feng F, Chu C, Yue W, Li L. A novel EIF4ENIF1 mutation associated with a diminished ovarian reserve and premature ovarian insufficiency identified by whole-exome sequencing. J Ovarian Res. 2019;12(1):119. https://doi.org/10.1186/s13048-019-0595-0.
Acknowledgements
The authors would like to thank Ania Pitas for the revision and correction of the language.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Morales, R., Lledo, B., Ortiz, J.A. et al. Identification of new variants and candidate genes in women with familial premature ovarian insufficiency using whole-exome sequencing. J Assist Reprod Genet 39, 2595–2605 (2022). https://doi.org/10.1007/s10815-022-02629-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-022-02629-3