Abstract
Purpose
To examine pregnancy outcomes after cryopreserved embryo transfer (ET) in breast cancer patients and to investigate the effect of controlled ovarian hyperstimulation (COH) as well as that of aromatase inhibitor (AI) administration and of the random start (RS) ovarian stimulation method.
Methods
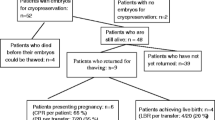
This retrospective study covered 126 patients who underwent embryo cryopreservation between 2010 and 2019. Thirty-one patients underwent frozen embryo transfer (FET), and we examined resulting pregnancy rates (PRs) and live birth rates (LBRs) in those who did and did not undergo COH and in relation to the AI and RS methods.
Results
PR and LBR per patient were higher among patients who underwent COH than among those who did not. PR per ET did not differ from that documented for non-cancer infertility patients, after adjustment for age. The PR and LBR did not differ between use and non-use of AI (27.8% vs 35.2%). In addition, there was no significant difference in the PR or LBR between RS and conventional start ovarian stimulation (33.3% vs 30.8%). No prenatal fetal abnormalities were observed in 8 cases (including 5 AI cases and 2 RS cases).
Conclusions
This study showed that the outcome of FET after FP was equivalent to that seen in non-cancer patients. Further, neither use of AI nor the RS method influenced LBR. COH including use of AI and the RS method are useful in FP for collecting and freezing many embryos within a short period and for increasing the per patient LBR after cancer treatment.


Similar content being viewed by others
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Change history
31 August 2022
A Correction to this paper has been published: https://doi.org/10.1007/s10815-022-02604-y
References
Phillips SM, Padgett LS, Leisenring WM, Stratton KK, Bishop K, Krull KR, et al. Survivors of childhood cancer in the United States: prevalence and burden of morbidity. Cancer Epidemiol Biomarkers Prev. 2015;24(4):653–63.
Donnez J, Dolmans MM. Fertility preservation in women. N Engl J Med. 2017;377(17):1657–65.
Cobo A, Garcia-Velasco J, Domingo J, Pellicer A, Remohi J. Elective and onco-fertility preservation: factors related to IVF outcomes. Human Reprod (Oxford, England). 2018;33(12):2222–31.
Cardozo ER, Thomson AP, Karmon AE, Dickinson KA, Wright DL, Sabatini ME. Ovarian stimulation and in-vitro fertilization outcomes of cancer patients undergoing fertility preservation compared to age matched controls: a 17-year experience. J Assist Reprod Genet. 2015;32(4):587–96.
Martinez F. Update on fertility preservation from the Barcelona International Society for Fertility Preservation-ESHRE-ASRM 2015 expert meeting: indications, results and future perspectives. Fertil Steril. 2017;108(3):407-15.e11.
Sonmezer M, Oktay K. Fertility preservation in young women undergoing breast cancer therapy. Oncologist. 2006;11(5):422–34.
Oktay K, Hourvitz A, Sahin G, Oktem O, Safro B, Cil A, et al. Letrozole reduces estrogen and gonadotropin exposure in women with breast cancer undergoing ovarian stimulation before chemotherapy. J Clin Endocrinol Metab. 2006;91(10):3885–90.
Ahmad MF, Sugishita Y, Suzuki-Takahashi Y, Sawada S, Iwahata H, Shiraishi E, et al. Oncofertility treatment among breast cancer women: a paradigm shift of practice after a decade of service. J Adolesc Young Adult Oncol. 2020;9(4):496–501.
Quinn MM, Cakmak H, Letourneau JM, Cedars MI, Rosen MP. Response to ovarian stimulation is not impacted by a breast cancer diagnosis. Human Reprod (Oxford, England). 2017;32(3):568–74.
Oktay K, Kim JY, Barad D, Babayev SN. Association of BRCA1 mutations with occult primary ovarian insufficiency: a possible explanation for the link between infertility and breast/ovarian cancer risks. J Clin Oncol: Off J Am Soc Clin Oncol. 2010;28(2):240–4.
Lambertini M, Goldrat O, Ferreira AR, Dechene J, Azim HA Jr, Desir J, et al. Reproductive potential and performance of fertility preservation strategies in BRCA-mutated breast cancer patients. Ann Oncol: Off J Eur Soc Med Oncol. 2018;29(1):237–43.
Porcu E, Cillo GM, Cipriani L, Sacilotto F, Notarangelo L, Damiano G, et al. Impact of BRCA1 and BRCA2 mutations on ovarian reserve and fertility preservation outcomes in young women with breast cancer. J Assist Reprod Genet. 2020;37(3):709–15.
Dolmans MM, de Ouderaen SH, Demylle D, Pirard C. Utilization rates and results of long-term embryo cryopreservation before gonadotoxic treatment. J Ass Reprod Gene. 2015;32(8):1233–7.
Oktay K, Turan V, Bedoschi G, Pacheco FS, Moy F. Fertility preservation success subsequent to concurrent aromatase inhibitor treatment and ovarian stimulation in women with breast cancer. J Clin Oncol: Off J Am Soc Clin Oncol. 2015;33(22):2424–9.
Tiboni GM. Aromatase inhibitors and teratogenesis. Fertil Steril. 2004;81(4):1158–9 (author reply 9).
Rodgers RJ, Reid GD, Koch J, Deans R, Ledger WL, Friedlander M, et al. The safety and efficacy of controlled ovarian hyperstimulation for fertility preservation in women with early breast cancer: a systematic review. Human Reprod (Oxford, England). 2017;32(5):1033–45.
Checa Vizcaíno MA, Corchado AR, Cuadri ME, Comadran MG, Brassesco M, Carreras R. The effects of letrozole on ovarian stimulation for fertility preservation in cancer-affected women. Reprod Biomed Online. 2012;24(6):606–10.
Johnson LN, Dillon KE, Sammel MD, Efymow BL, Mainigi MA, Dokras A, et al. Response to ovarian stimulation in patients facing gonadotoxic therapy. Reprod Biomed Online. 2013;26(4):337–44.
Bercaire LMN, Cavagna M, Donadio NF, Rocha AR, Portela R, Alves VR, et al. The impact of letrozole administration on oocyte morphology in breast cancer patients undergoing fertility preservation. JBRA assisted reproduction. 2020;24(3):257–64.
Goldrat O, Van Den Steen G, Gonzalez-Merino E, Dechene J, Gervy C, Delbaere A, et al. Letrozole-associated controlled ovarian hyperstimulation in breast cancer patients versus conventional controlled ovarian hyperstimulation in infertile patients: assessment of oocyte quality related biomarkers. Reprod Biol Endocrinol. 2019;17(1):3.
Cakmak H, Katz A, Cedars MI, Rosen MP. Effective method for emergency fertility preservation: random-start controlled ovarian stimulation. Fertil Steril. 2013;100(6):1673–80.
Campos APC, Geber GP, Hurtado R, Sampaio M, Geber S. Ovarian response after random-start controlled ovarian stimulation to cryopreserve oocytes in cancer patients. JBRA Ass Reprod. 2018;22(4):352–4.
Nakasuji T, Kawai K, Ishikawa T, Teraoka K, Takeuchi S, Miyagawa T, et al. Random-start ovarian stimulation with aromatase inhibitor for fertility preservation in women with Japanese breast cancer. Reprod Med Biol. 2019;18(2):167–72.
Filippi F, Somigliana E, Busnelli A, Guarneri C, Noli S, Restelli L, et al. The presence of dominant follicles and corpora lutea does not perturb response to controlled ovarian stimulation in random start protocols. Sci Rep. 2020;10(1):10083.
Vaiarelli A, Cimadomo D, Alviggi E, Sansone A, Trabucco E, Dusi L, et al. The euploid blastocysts obtained after luteal phase stimulation show the same clinical, obstetric and perinatal outcomes as follicular phase stimulation-derived ones: a multicenter study. Human Reprod (Oxford, England). 2020;35(11):2598–608.
Acknowledgements
The authors thank all the staff at St. Marianna University Hospital Reproductive Center for their support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This retrospective study was approved by the research ethics committee of St. Marianna University School of Medicine.
Consent to participate
This study is a retrospective study. Patients were not required to give informed consent to the study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The correct name of the 4th author is Yodo Sugishita instead of Yodo Sugisita.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Okutsu-Horage, Y., Iwahata, H., Suzuki-Takahashi, Y. et al. Clinical outcome of embryo cryopreservation in Japanese breast cancer patients: pregnancy rates after transfer of thawed embryos. J Assist Reprod Genet 39, 1769–1777 (2022). https://doi.org/10.1007/s10815-022-02575-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-022-02575-0