Abstract
Purpose
To compare ovarian response and the number of transferable embryos between women with balanced autosomal translocations and women whose partners carry the translocation (control group). To investigate the predictive value of metaphase II (MII) oocyte number and biopsied embryo number for gaining at lowest one transferable embryo.
Design
We retrospectively analyzed 1942 preimplantation genetic testing for structural rearrangements (PGT-SR) cycles of 1505 balanced autosomal translocation couples over 8 years. All cycles were divided into two subgroups: Robertsonian and reciprocal translocations (ROBT and ReBT). Receiver operator characteristic (ROC) curves were plotted to ascertain a cutoff of MII oocytes and biopsied embryos as predictors of gaining at lowest one transferable embryo.
Result
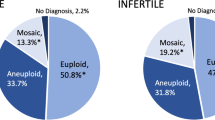
There were no statistical differences in baseline features or ovarian response indicators regarding the number of retrieved/MII oocytes, E2 level on the day of HCG, and ovarian sensitivity index (OSI) between women with balanced autosomal translocations and control group (P > 0.05). A decreased number of transferable embryos were found in women with balanced autosomal translocations regardless of the type of translocation. The cutoff values for gaining at lowest one transferable embryo are 12.5 MII oocytes and 4.5 biopsied embryos, respectively.
Conclusion
Women with balanced autosomal translocations have a normal ovarian response, but fewer transferable embryos, meaning that higher gonadotropin (Gn) doses may be required to increase transferable embryos. When fewer than 12.5 MII oocytes or 4.5 blastocysts are obtained in a PGT-SR cycle, couples should be notified that the likelihood of gaining a transferable embryo is low.


Similar content being viewed by others
References
Hamerton JL, Canning N, Ray M, Smith S. A cytogenetic survey of 14069 newborn infants. I. Incidence of chromosome abnormalities. Clin Genet. 1975;8(4):223–43. https://doi.org/10.1111/j.1399-0004.1975.tb01498.x.
Fryns JP, Van Buggenhout G. Structural chromosome rearrangements in couples with recurrent fetal wastage. Eur J Obstet Gynecol Reprod Biol. 1998;81(2):171–6. https://doi.org/10.1016/s0301-2115(98)00185-7.
Scriven PN. PGT-SR (reciprocal translocation) using trophectoderm sampling and next-generation sequencing: insights from a virtual trial. J Assist Reprod Genet. 2021;38(8):1971–8. https://doi.org/10.1007/s10815-021-02174-5.
Mayeur A, Ahdad N, Hesters L, Grynberg M, Romana S, Sonigo C, et al. Does the prognosis after PGT for structural rearrangement differ between female and male translocation carriers? Reprod Biomed Online. 2020;40(5):684–92. https://doi.org/10.1016/j.rbmo.2020.01.025.
Tong J, Niu Y, Wan A, Zhang T. Effect of parental origin and predictors for obtaining a euploid embryo in balanced translocation carriers. Reprod Biomed Online. 2021. https://doi.org/10.1016/j.rbmo.2021.09.007.
Chen SH, Escudero T, Cekleniak NA, Sable DB, Garrisi MG, Munne S. Patterns of ovarian response to gonadotropin stimulation in female carriers of balanced translocation. Fertil Steril. 2005;83(5):1504–9. https://doi.org/10.1016/j.fertnstert.2004.11.058.
Lledó B, Ortiz JA, Morales R, Ten J, de la Fuente PE, García-Ochoa C, et al. The paternal effect of chromosome translocation carriers observed from meiotic segregation in embryos. Hum Reprod. 2010;25(7):1843–8. https://doi.org/10.1093/humrep/deq111.
Harper JC, Wilton L, Traeger-Synodinos J, Goossens V, Moutou C, SenGupta SB, et al. The ESHRE PGD Consortium: 10 years of data collection. Hum Reprod Update. 2012;18(3):234–47. https://doi.org/10.1093/humupd/dmr052.
Mateu-Brull E, Rodrigo L, Peinado V, Mercader A, Campos-Galindo I, Bronet F, et al. Interchromosomal effect in carriers of translocations and inversions assessed by preimplantation genetic testing for structural rearrangements (PGT-SR). J Assist Reprod Genet. 2019;36(12):2547–55. https://doi.org/10.1007/s10815-019-01593-9.
Tur-Kaspa I. Clinical management of in vitro fertilization with preimplantation genetic diagnosis. Semin Reprod Med. 2012;30(4):309–22. https://doi.org/10.1055/s-0032-1313910.
Huber M, Hadziosmanovic N, Berglund L, Holte J. Using the ovarian sensitivity index to define poor, normal, and high response after controlled ovarian hyperstimulation in the long gonadotropin-releasing hormone-agonist protocol: suggestions for a new principle to solve an old problem. Fertil Steril. 2013;100(5):1270–6. https://doi.org/10.1016/j.fertnstert.2013.06.049.
Li HW, Lee VC, Ho PC, Ng EH. Ovarian sensitivity index is a better measure of ovarian responsiveness to gonadotrophin stimulation than the number of oocytes during in-vitro fertilization treatment. J Assist Reprod Genet. 2014;31(2):199–203. https://doi.org/10.1007/s10815-013-0144-5.
Niu W, Wang L, Xu J, Li Y, Shi H, Li G, et al. Improved clinical outcomes of preimplantation genetic testing for aneuploidy using MALBAC-NGS compared with MDA-SNP array. BMC pregnancy childbirth. 2020;20(1):388. https://doi.org/10.1186/s12884-020-03082-9.
Dechanet C, Castelli C, Reyftmann L, Hamamah S, Hedon B, Dechaud H, et al. Do female translocations influence the ovarian response pattern to controlled ovarian stimulation in preimplantation genetic diagnosis? Hum Reprod. 2011;26(5):1232–40. https://doi.org/10.1093/humrep/der032.
Alviggi C, Conforti A, Esteves SC, Vallone R, Venturella R, Staiano S, et al. Understanding ovarian hypo-response to exogenous gonadotropin in ovarian stimulation and its new proposed marker-the follicle-to-oocyte (FOI) index. Front Endocrinol. 2018;9:589. https://doi.org/10.3389/fendo.2018.00589.
Verdoni A, Hu J, Surti U, Babcock M, Sheehan E, Clemens M, et al. Reproductive outcomes in individuals with chromosomal reciprocal translocations. Genet Med. 2021;23(9):1753–60. https://doi.org/10.1038/s41436-021-01195-w.
Ko DS, Cho JW, Lee HS, Kim JY, Kang IS, Yang KM, et al. Preimplantation genetic diagnosis outcomes and meiotic segregation analysis of robertsonian translocation carriers. Fertil Steril. 2013;99(5):1369–76. https://doi.org/10.1016/j.fertnstert.2012.12.010.
Liu H, Mao B, Xu X, Liu L, Ma X, Zhang X. The effectiveness of next-generation sequencing-based preimplantation genetic testing for balanced translocation couples. Cytogenet Genome Res. 2020;160(11–12):625–33. https://doi.org/10.1159/000512847.
Ma X, Xu X, Mao B, Liu H, Li H, Liu K, et al. Chromosomal analysis for embryos from balanced chromosomal rearrangement carriers using next generation sequencing. Mol Reprod Dev. 2021;88(5):362–70. https://doi.org/10.1002/mrd.23469.
Bint SM, Ogilvie CM, Flinter FA, Khalaf Y, Scriven PN. Meiotic segregation of Robertsonian translocations ascertained in cleavage-stage embryos–implications for preimplantation genetic diagnosis. Hum Reprod. 2011;26(6):1575–84. https://doi.org/10.1093/humrep/der080.
Song H, Shi H, Yang ET, Bu ZQ, Jin ZQ, Huo MZ, et al. Effects of gender of reciprocal chromosomal translocation on blastocyst formation and pregnancy outcome in preimplantation genetic testing. Front Endocrinol. 2021;12:704299. https://doi.org/10.3389/fendo.2021.704299.
Lin L, Chen X, Wang J, Li R, Ding C, Cai B, et al. Effect of carriers’ sex on meiotic segregation patterns and chromosome stability of reciprocal translocations. Reprod Biomed Online. 2021;43(6):1011–8. https://doi.org/10.1016/j.rbmo.2021.08.017.
Zhang S, Lei C, Wu J, Sun H, Zhou J, Zhu S, et al. Analysis of segregation patterns of quadrivalent structures and the effect on genome stability during meiosis in reciprocal translocation carriers. Hum Reprod. 2018;33(4):757–67. https://doi.org/10.1093/humrep/dey036.
Mackie Ogilvie C, Scriven PN. Meiotic outcomes in reciprocal translocation carriers ascertained in 3-day human embryos. Eur J Hum Genet. 2002;10(12):801–6. https://doi.org/10.1038/sj.ejhg.5200895.
Katagiri Y, Tamaki Y. Genetic counseling prior to assisted reproductive technology. Reprod Med Biol. 2021;20(2):133–43. https://doi.org/10.1002/rmb2.12361.
Zhang L, Wei D, Zhu Y, Jiang W, Xia M, Li J, et al. Interaction of acrocentric chromosome involved in translocation and sex of the carrier influences the proportion of alternate segregation in autosomal reciprocal translocations. Hum Reprod. 2019;34(2):380–7. https://doi.org/10.1093/humrep/dey367.
Lei C, Zhang S, Zhu S, Wu J, Xiao M, Zhou J, et al. Conventional ICSI improves the euploid embryo rate in male reciprocal translocation carriers. J Assist Reprod Genet. 2021;38(1):129–38. https://doi.org/10.1007/s10815-020-02013-z.
Wang YZ, Ding CH, Wang J, Zeng YH, Zhou W, Li R, et al. Number of blastocysts biopsied as a predictive indicator to obtain at least one normal/balanced embryo following preimplantation genetic diagnosis with single nucleotide polymorphism microarray in translocation cases. J Assist Reprod Genet. 2017;34(1):51–9. https://doi.org/10.1007/s10815-016-0831-0.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present study was consistent with the Declaration of Helsinki and received approval from the Research Ethics Board of the First Affiliated Hospital of Zhengzhou University.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, M., Bu, Z., Liu, Y. et al. Are ovarian responses and the number of transferable embryos different in females and partners of male balanced translocation carriers?. J Assist Reprod Genet 39, 2019–2026 (2022). https://doi.org/10.1007/s10815-022-02563-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-022-02563-4