Abstract
Purpose
This study aims to investigate whether indomethacin (IND) delays preterm birth by regulating the Notch pathway, Tlr receptors, and Sp-A in the placenta in lipopolysaccharide (LPS)-induced preterm labor (PTL) model.
Methods
CD-1 mice were distributed to the pregnant control (PC), Sham, PBS, IND (2 mg/kg; i.p.), LPS (25 μg/100 μl; intrauterine), and LPS + IND groups. The injections were performed on day 14.5 of pregnancy. Placentae were collected on day 15.5 of pregnancy, and immunohistochemical analyzes were performed. Differences in staining intensities between the Cox-1, Notch-1 (N1), Dll-1, Jagged-2 (Jag-2), Tlr-2, and Tlr-4 proteins were compared.
Results
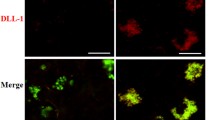
Preterm labor rates were 100% and 66% (preterm delivery delayed 5 h) in the LPS and LPS + IND groups, respectively. In LPS-treated mice, a general morphological deterioration was observed in the placenta. Total placental mid-sagittal measurement was significantly reduced in the LPS-treated group, while it was similar to the PC group in the LPS + IND group. Cox-1 expression in the LZ increased, and Sp-A expression decreased after LPS injection, and IND administration diminished this increase. N1 expression increased in the labyrinth zone (LZ) and the junctional zone (JZ). Dll-1 and Jag-2 expression increased in the JZ after LPS injection (p < 0.0001). IND administration diminished Tlr-2 expression in the LZ and Tlr-4 expression in the JZ after LPS injection.
Conclusion
In conclusion, PG (prostaglandin) inhibition may alter Notch signaling, Tlr, and Sp-A protein expression and may be associated with delayed labor in LPS-induced mice.









Similar content being viewed by others
Data availability
All data will be provided by the corresponding author upon request.
References
Furuya M, et al. Pathophysiology of placentation abnormalities in pregnancy-induced hypertension. Vasc Health Risk Manag. 2008;4(6):1301–13.
Maltepe E, Fisher SJ. Placenta: the forgotten organ. Annu Rev Cell Dev Biol. 2015;31:523–52.
Fisher SJ. The placenta dilemma. Semin Reprod Med. 2000;18(3):321–6.
Isaac SM, et al. In: Anne Croy B, et al., editors. Anatomy of the mouse placenta throughout gestation, in the guide to investigation of mouse pregnancy: Academic Press; 2014. p. 69–74.
Azevedo Portilho N, Pelajo-Machado M. Mechanism of hematopoiesis and vasculogenesis in mouse placenta. Placenta. 2018;69:140–5.
Azevedo Portilho N, et al. Localization of transient immature hematopoietic cells to two distinct, potential niches in the developing mouse placenta. Placenta. 2016;47:1–11.
Hu D, Cross JC. Development and function of trophoblast giant cells in the rodent placenta. Int J Dev Biol. 2010;54(2-3):341–54.
Simmons DG, Fortier AL, Cross JC. Diverse subtypes and developmental origins of trophoblast giant cells in the mouse placenta. Dev Biol. 2007;304(2):567–78.
Hemberger M, Hanna CW, Dean W. Mechanisms of early placental development in mouse and humans. Nat Rev Genet. 2020;21(1):27–43.
Bolon B. In: Anne Croy B, et al., editors. Pathology analysis of the placenta, in The guide to investigation of mouse pregnancy: Elsevier Inc.; 2014. p. 175–88.
De Falco M, et al. Expression and distribution of notch protein members in human placenta throughout pregnancy. Placenta. 2007;28(2-3):118–26.
Hunkapiller NM, et al. A role for Notch signaling in trophoblast endovascular invasion and in the pathogenesis of pre-eclampsia. Development. 2011;138(14):2987–98.
Gasperowicz M, Otto F. The notch signalling pathway in the development of the mouse placenta. Placenta. 2008;29(8):651–9.
Zhao WX, Lin JH. Notch signaling pathway and human placenta. Int J Med Sci. 2012;9(6):447–52.
Walker L, et al. The notch receptor and its ligands are selectively expressed during hematopoietic development in the mouse. Stem Cells. 2001;19(6):543–52.
Gasperowicz M, Rai A, Cross JC. Spatiotemporal expression of Notch receptors and ligands in developing mouse placenta. Gene Expr Patterns. 2013;13(7):249–54.
Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7(9):678–89.
Keewan E, Naser SA. The role of notch signaling in macrophages during inflammation and infection: implication in rheumatoid arthritis? Cells. 2020;9(1).
Shang Y, Smith S, Hu X. Role of Notch signaling in regulating innate immunity and inflammation in health and disease. Protein Cell. 2016;7(3):159–74.
Agrawal V, et al. Role of Notch signaling during lipopolysaccharide-induced preterm labor. J Leukoc Biol. 2016;100(2):261–74.
Bollapragada S, et al. Term labor is associated with a core inflammatory response in human fetal membranes, myometrium, and cervix. Am J Obstet Gynecol. 2009;200(1):104–e1-11.
Boyle AK, et al. Preterm birth: inflammation, fetal injury and treatment strategies. J Reprod Immunol. 2017;119:62–6.
Eloundou SN, et al. Placental malperfusion in response to intrauterine inflammation and its connection to fetal sequelae. PLoS One. 2019;14(4):e0214951.
Renaud SJ, et al. Spontaneous pregnancy loss mediated by abnormal maternal inflammation in rats is linked to deficient uteroplacental perfusion. J Immunol. 2011;186(3):1799–808.
Timmons BC, et al. Prostaglandins are essential for cervical ripening in LPS-mediated preterm birth but not term or antiprogestin-driven preterm ripening. Endocrinology. 2014;155(1):287–98.
Rankin JG. A role for prostaglandins in the regulation of the placental blood flows. Prostaglandins. 1976;11(2):343–53.
Rhind SG, et al. Indomethacin inhibits circulating PGE2 and reverses postexercise suppression of natural killer cell activity. Am J Phys. 1999;276(5):R1496–505.
Snegovskikh VV, et al. Surfactant protein-A (SP-A) selectively inhibits prostaglandin F2alpha (PGF2alpha) production in term decidua: implications for the onset of labor. J Clin Endocrinol Metab. 2011;96(4):E624–32.
Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5(1):58–68.
Agrawal V, et al. Surfactant protein (SP)-A suppresses preterm delivery and inflammation via TLR2. PLoS One. 2013;8(5):e63990.
Sones JL, Davisson RL. Preeclampsia, of mice and women. Physiol Genomics. 2016;48(8):565–72.
Zhang YH, et al. Modulators of the balance between M1 and M2 macrophages during pregnancy. Front Immunol. 2017;8:120.
Altshuler G, et al. Premature onset of labor, neonatal patent ductus arteriosus, and prostaglandin synthetase antagonists--a rat model of a human problem. Am J Obstet Gynecol. 1979;135(2):261–5.
Lai JH, et al. A randomized trial comparing the efficacy of single-dose and double-dose administration of rectal indomethacin in preventing post-endoscopic retrograde cholangiopancreatography pancreatitis. Medicine (Baltimore). 2019;98(20):e15742.
Huang H, et al. Sensitivity of mice to lipopolysaccharide is increased by a high saturated fat and cholesterol diet. J Inflamm (Lond). 2007;4:22.
Grigsby PL, et al. Fetal responses to maternal and intra-amniotic lipopolysaccharide administration in sheep. Biol Reprod. 2003;68(5):1695–702.
Kim HS, et al. Endotoxin-neutralizing antimicrobial proteins of the human placenta. J Immunol. 2002;168(5):2356–64.
Xue H, et al. Indomethacin inhibits PGE2, regulates inflammatory response, participates in adipogenesis regulation, and improves success rate of fat transplantation in C57/B6 mice. Trop J Pharm Res. 2019;18(11):2313–8.
Charlier C, Michaux C. Dual inhibition of cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX) as a new strategy to provide safer non-steroidal anti-inflammatory drugs. Eur J Med Chem. 2003;38(7-8):645–59.
Gross G, et al. Inhibition of cyclooxygenase-2 prevents inflammation-mediated preterm labor in the mouse. Am J Phys Regul Integr Comp Phys. 2000;278(6):R1415–23.
Gross GA, et al. Opposing actions of prostaglandins and oxytocin determine the onset of murine labor. Proc Natl Acad Sci U S A. 1998;95(20):11875–9.
Benedito R, et al. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137(6):1124–35.
Blanco R, Gerhardt H. VEGF and Notch in tip and stalk cell selection. Cold Spring Harb Perspect Med. 2013;3(1):a006569.
Boareto M, et al. Jagged mediates differences in normal and tumor angiogenesis by affecting tip-stalk fate decision. Proc Natl Acad Sci U S A. 2015;112(29):E3836–44.
Tunster SJ, et al. Placental glycogen stores and fetal growth: insights from genetic mouse models. Reproduction. 2020;159(6):R213–35.
Kim W, et al. Notch signaling in pancreatic endocrine cell and diabetes. Biochem Biophys Res Commun. 2010;392(3):247–51.
Moco NP, et al. Gene expression and protein localization of TLR-1, -2, -4 and -6 in amniochorion membranes of pregnancies complicated by histologic chorioamnionitis. Eur J Obstet Gynecol Reprod Biol. 2013;171(1):12–7.
Kim YM, et al. Toll-like receptor-2 and -4 in the chorioamniotic membranes in spontaneous labor at term and in preterm parturition that are associated with chorioamnionitis. Am J Obstet Gynecol. 2004;191(4):1346–55.
Jing X, et al. Toll-like receptor 2/4 inhibitors can reduce preterm birth in mice. J Int Med Res. 2020;48(10):300060520933795.
Henning LN, et al. Pulmonary surfactant protein A regulates TLR expression and activity in human macrophages. J Immunol. 2008;180(12):7847–58.
Borron P, et al. Surfactant-associated protein A inhibits LPS-induced cytokine and nitric oxide production in vivo. Am J Phys Lung Cell Mol Phys. 2000;278(4):L840–7.
LeVine AM, Whitsett JA. Pulmonary collectins and innate host defense of the lung. Microbes Infect. 2001;3(2):161–6.
Funding
SA was funded by the Scientific and Technological Research Council of Turkey with International Research Fellowship 2214-A Programme for PhD students grant (1059B141700505-2214/A. The study represents a part of the PhD thesis of SA.
Author information
Authors and Affiliations
Contributions
SA designed the study, performed the experimental procedures, analyzed the data, and drafted the manuscript. NK, BD, and LK assisted SA during experiments, surgical procedures, and data generation. IU acted as a consultant during the execution of the project. CCO contributed to the data analysis and drafting of the article.
Corresponding author
Ethics declarations
Ethics approval
The Animal Research Ethical Committee approved the experimental protocol of Akdeniz University by protocol number 1201/2020.10.008. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Consent to participate
All authors have read and accepted the content of the article; all authors have their consent to participate in the study.
Consent for publication
All authors have consent for the publication of this article.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Part of the study has been presented as a poster presentation at the 77th American Society for Reproductive Medicine Scientific Congress that has been held in Baltimore in person and on-demand between 17–20 October 2021.
Rights and permissions
About this article
Cite this article
Avci, S., Kuscu, N., Durkut, B. et al. Altered expression of Notch signaling, Tlr receptors, and surfactant protein expression after prostaglandin inhibition may be associated with the delayed labor in LPS-induced mice. J Assist Reprod Genet 39, 1531–1544 (2022). https://doi.org/10.1007/s10815-022-02515-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-022-02515-y