Abstract
Purpose
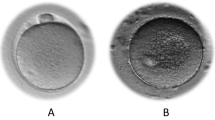
Spontaneous oocyte activation (SOA) is a recently classified phenomenon characterized by the presence of a single pronucleus immediately following oocyte retrieval, without the apparent involvement of sperm. SOA currently remains poorly understood in humans, with no clear genetic or pathological factor(s). Herein, we report two separate cases of recurrent spontaneous oocyte activation, investigating potential avenues to identify causative etiology.
Methods
Two patients with several cycles with SOA have undergone further genetic and embryologic investigation to reveal underlying causes for SOA and provide a treatment if possible.
Results
One case was a patient with recurrent pregnancy loss and the other was diagnosed as unexplained infertility. In the first case, 61 out of 69 oocytes retrieved exhibited SOA in five cycles while in the second case 44 out of 49 oocytes exhibited SOA in five cycles. Oocytes were injected with sperm; embryo development and presence of paternal contribution were investigated. No pregnancy is ensued following embryo transfer in both patients. Time-lapse imaging of embryogenesis from the second case did not reveal even momentary second pronucleus appearance. We also performed clinical whole exome sequencing for both patients but did not identify any disease-causing variant.
Conclusion
Patients with SOA suffer from infertility. Our results indicate that more investigation is required to understand the etiology of SOA in humans concentrating on the molecular mechanisms that underpin regulation of oocyte activation and calcium dynamics need to be investigated to fully understand, and perhaps in the future rectify, recurrent SOA.

Similar content being viewed by others
References
Coticchio G, Dal Canto M, Mignini Renzini M, Guglielmo MC, Brambillasca F, Turchi D et al. Oocyte maturation: gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization. Hum Reprod Update. 21(4):427–54. https://doi.org/10.1093/humupd/dmv011.
Okabe M. The cell biology of mammalian fertilization. Development. 2013;140(22):4471–9. https://doi.org/10.1242/dev.090613.
Kashir J. Increasing associations between defects in phospholipase C zeta and conditions of male infertility: not just ICSI failure? J Assist Reprod Genet. 2020;37(6):1273–93. https://doi.org/10.1007/s10815-020-01748-z.
Saleh A, Kashir J, Thanassoulas A, Safieh-Garabedian B, Lai FA, Nomikos M. Essential role of sperm-specific PLC-Zeta in egg activation and male factor infertility: an update. Front Cell Dev Biol. 2020;29(8):28. https://doi.org/10.3389/fcell.2020.00028.
Sen A, Caiazza F. Oocyte maturation: a story of arrest and release. Front Biosci (Schol Ed). 2013;1(5):451–77. https://doi.org/10.2741/s383.
Tiwari M, Gupta A, Sharma A, Prasad S, Pandey AN, Yadav PK, et al. Role of mitogen activated protein kinase and maturation promoting factor during the achievement of meiotic competency in mammalian oocytes. J Cell Biochem. 2018;119:123–9. https://doi.org/10.1002/jcb.26184.
Cui W. Oocyte spontaneous activation: an overlooked cellular event that impairs female fertility in mammals. Front Cell Dev Biol. 2021;8(9): 648057. https://doi.org/10.3389/fcell.2021.648057.
Madgwick S, Jones KT. How eggs arrest at metaphase II: MPF stabilisation plus APC/C inhibition equals cytostatic factor. Cell Div. 2007;26(2):4. https://doi.org/10.1186/1747-1028-2-4.
Kito S, Yano H, Ohta Y, Tsukamoto S. Superovulatory response, oocyte spontaneous activation, and embryo development in WMN/Nrs inbred rats. Exp Anim. 2010;59:35–45. https://doi.org/10.1538/expanim.59.35.
Zernicka-Goetz M. Spontaneous and induced activation of rat oocytes. Mol Reprod Dev. 1991;28(2):169–76. https://doi.org/10.1002/mrd.1080280210.
Chebotareva T, Taylor J, Mullins JJ, Wilmut I. Rat eggs cannot wait: Spontaneous exit from meiotic metaphase-II arrest. Mol Reprod Dev. 2011;78(10–11):795–807. https://doi.org/10.1002/mrd.21385.
Hashimoto N, Watanabe N, Furuta Y, Tamemoto H, Sagata N, Yokoyama M, et al. Parthenogenetic activation of oocytes in c-mos-deficient mice. Nature. 1994;370(6484):68–71. https://doi.org/10.1038/370068a0.
Combelles CM, Kearns WG, Fox JH, Racowsky C. Cellular and genetic analysis of oocytes and embryos in a human case of spontaneous oocyte activation. Hum Reprod. 2011;26(3):545–52. https://doi.org/10.1093/humrep/deq363.
Ye Y, Li N, Yan X, Wu R, Zhou W, Cheng L, et al. Genetic analysis of embryo in a human case of spontaneous oocyte activation: a case report. Gynecol Endocrinol. 2020;36:294–6. https://doi.org/10.1080/09513590.2019.
Osman EK, Hong KH, Scott RT. A case of recurrent spontaneous parthenogenetic oocyte activation. Reprod Biomed Online. 2019;39(Suppl. 2):e7–8.
Qubbaj W, Al-Swaid A, Al-Hassan S, Awartani K, Deek H, Coskun S. First successful application of preimplantation genetic diagnosis and haplotyping for congenital hyperinsulinism. Reprod Biomed Online. 2011;22(1):72–9. https://doi.org/10.1016/j.rbmo.2010.09.016.
Alghofaili L, Almubarak H, Gassem K, Islam SS, Coskun S, Kaya N, et al. Cell-free DNA levels of twins and sibling pairs indicate individuality and possible use as a personalized biomarker. PLoS ONE. 2019;14(10): e0223470. https://doi.org/10.1371/journal.pone.0223470.
Monies D, Abouelhoda M, AlSayed M, Alhassnan Z, Alotaibi M, Kayyali H, et al. The landscape of genetic diseases in Saudi Arabia based on the first 1000 diagnostic panels and exomes. Hum Genet. 2017;136(8):921–39. https://doi.org/10.1007/s00439-017-1821-8.
Monies D, Abouelhoda M, Assoum M, Moghrabi N, Rafiullah R, Almontashiri N, et al. Lessons learned from large-scale, first-tier clinical exome sequencing in a highly consanguineous population. Am J Hum Genet. 2019;104(6):1182–201. https://doi.org/10.1016/j.ajhg.2019.04.011.
Jaroudi K, Al-Hassan S, Al-Sufayan H, Al-Mayman H, Qeba M, Coskun S. Intracytoplasmic sperm injection and conventional in vitro fertilization are complementary techniques in management of unexplained infertility. J Assist Reprod Genet. 2003;20(9):377–81. https://doi.org/10.1023/a:1025433128518.
Zhang YL, Liu XM, Ji SY, Sha QQ, Zhang J, Fan HY. ERK1/2 activities are dispensable for oocyte growth but are required for meiotic maturation and pronuclear formation in mouse. J Genet Genomics. 2015;42(9):477–85. https://doi.org/10.1016/j.jgg.2015.07.004.
Altaf S, Bao J. Exome sequencing shines in empty follicle syndrome: zona pellucida gene mutations manifest genuine empty follicle syndrome. Fertil Steril. 2021;115(5):1170–1. https://doi.org/10.1016/j.fertnstert.2021.03.007.
Maddirevula S, Awartani K, Coskun S, AlNaim LF, Ibrahim N, Abdulwahab F, et al. A genomics approach to females with infertility and recurrent pregnancy loss. Hum Genet. 2020;139(5):605–13. https://doi.org/10.1007/s00439-020-02143-5.
Yeste M, Jones C, Amdani SN, Patel S, Coward K. Oocyte activation deficiency: a role for an oocyte contribution? Hum Reprod Update. 2016;22(1):23–47. https://doi.org/10.1093/humupd/dmv040.
Cui W, Zhang J, Lian HY, Wang HL, Miao DQ, Zhang CX, et al. Roles of MAPK and spindle assembly checkpoint in spontaneous activation and MIII arrest of rat oocytes. PLoS ONE. 2012;7(2): e32044. https://doi.org/10.1371/journal.pone.0032044.
Asami M, Lam BYH, Ma MK, Rainbow K, Braun S, VerMilyea MD, et al. Human embryonic genome activation initiates at the one-cell stage. Cell Stem Cell. 2022;29(2):209-216.e4. https://doi.org/10.1016/j.stem.2021.11.012.
Kim AM, Bernhardt ML, Kong BY, Ahn RW, Vogt S, Woodruff TK, et al. Zinc sparks are triggered by fertilization and facilitate cell cycle resumption in mammalian eggs. ACS Chem Biol. 2011;6(7):716–23. https://doi.org/10.1021/cb200084y.
Duncan FE, Que EL, Zhang N, Feinberg EC, O’Halloran TV, Woodruff TK. The zinc spark is an inorganic signature of human egg activation. Sci Rep. 2016;26(6):24737. https://doi.org/10.1038/srep24737.
Sun L, Chai Y, Hannigan R, Bhogaraju VK, Machaca K. Zinc regulates the ability of Cdc25C to activate MPF/cdk1. J Cell Physiol. 2007;213(1):98–104. https://doi.org/10.1002/jcp.21090.
Madgwick S, Hansen DV, Levasseur M, Jackson PK, Jones KT. Mouse Emi2 is required to enter meiosis II by reestablishing cyclin B1 during interkinesis. J Cell Biol. 2006;174(6):791–801. https://doi.org/10.1083/jcb.200604140.
Lee K, Davis A, Zhang L, Ryu J, Spate LD, Park KW, Samuel MS, Walters EM, Murphy CN, Machaty Z, Prather RS. Pig oocyte activation using a Zn2+ chelator, TPEN. Theriogenology. 2015;84(6):1024–32. https://doi.org/10.1016/j.theriogenology.2015.05.036.
Kashir & Swann, Chapter 14: Assisted oocyte activation: Current understanding, practice, and future perspectives, 219–234. In: Textbook of Assisted Reproductive Techniques, David K. Gardner, Ariel Weissman, Colin M. Howles, Zeev Shoham (Eds), 5th edition, CRC Press, Boca Raton.
Author information
Authors and Affiliations
Contributions
SC and FA conceived the study. SC and JK wrote the manuscript. KA and MA recruited the patients and collected data. WQ and SM performed the experiments. All authors contributed to the article and approved the submitted version.
The authors declare no financial or non-financial interests that are directly or indirectly related to the work submitted for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (AVI 2144 KB)
10815_2022_2435_MOESM2_ESM.jpg
Figure S1. Exome was performed on index patients from case 1 and 2. Filtering strategy starting from larger to smaller circles; Total number of variants=>Homozygous variants=>Coding/Splicing=>Absent in gnomAD and local database=>Predicted deleterious in silico=>Relevant biology (JPG 110 KB)
Supplementary Video 1. Development of embryo generated from the spontaneously activated oocyte following ICSI. Although STR analysis showed paternal contribution, no other pronucleus was seen. (AVI 18404 KB)
Rights and permissions
About this article
Cite this article
Coskun, S., Maddirevula, S., Awartani, K. et al. Recurrent spontaneous oocyte activation causes female infertility. J Assist Reprod Genet 39, 675–680 (2022). https://doi.org/10.1007/s10815-022-02435-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-022-02435-x