Abstract
Purpose
This study was conducted to verify if the cfDNA integrity (cfDI) in follicular fluid and subsequent spent embryo medium (SEM) could serve as potential non-invasive biomarker for high-grade embryo selection during IVF/ICSI.
Methods
Thirty-two follicular fluids, 32 subsequent corresponding cleavage embryo SEM, and 23 subsequent blastocyst SEM were collected from 11 patients undergoing IVF/ICSI. CfDI was measured by ALU gene amplicons with different sizes by qPCR, as the ratio of long to short fragments.
Results
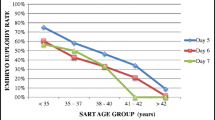
CfDI in follicular fluid corresponding to subsequent high-grade cleavage embryos and blastocysts was significantly lower than that related to low-grade embryos (p = 0.018). Conversely, cfDI in SEM was significantly and positively correlated with high-grade embryos at both stages (p = 0.009). ROC curves of the analysis of cfDI in follicular fluid showed great potential in predicting subsequent embryogenesis and embryo grade (AUC > 0.927). Regardless of the cleavage embryo grade by morphology, cfDI in day 3 SEM could predict if the cleavage embryo could develop to a high-grade blastocyst (AUC = 0.820). A concordant shift pattern of cfDI from follicular fluid to subsequent day 3 SEM and day 5 SEM was found in 81.82% participants featured by various clinical characteristics.
Conclusion
CfDI in follicular fluid and SEM was significantly correlated with embryogenesis and embryo grade and could serve as a potential non-invasive biomarker in high-grade embryo selection. Direct qPCR was proved as a labor-saving and sensitive method for the analysis of cfDI in low volume of SEM.








Similar content being viewed by others
References
Guerif F, Le Gouge A, Giraudeau B, Poindron J, Bidault R, Gasnier O, Royere D. Limited value of morphological assessment at days 1 and 2 to predict blastocyst development potential: a prospective study based on 4042 embryos. Hum Reprod. 2007;22(7):1973–81.
Canick JA, Palomaki GE, Kloza EM, Lambert-Messerlian GM, Haddow JE. The impact of maternal plasma DNA fetal fraction on next generation sequencing tests for common fetal aneuploidies. Prenat Diagn. 2013;33(7):667–74.
Galeva S, Gil MM, Konstantinidou L, Akolekar R, Nicolaides KH. First-trimester screening for trisomies by cfDNA testing of maternal blood in singleton and twin pregnancies: factors affecting test failure. Ultrasound in Obstetrics & Gynecology : the Official Journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2019;53(6):804–9.
Chan KC, Leung SF, Yeung SW, Chan AT, Lo YM. Persistent aberrations in circulating DNA integrity after radiotherapy are associated with poor prognosis in nasopharyngeal carcinoma patients. Clinical Cancer Research : an Official Journal of the American Association for Cancer Research. 2008;14(13):4141–5.
Vergouw CG, Botros LL, Roos P, Lens JW, Schats R, Hompes PG, Burns DH, Lambalk CB. Metabolomic profiling by near-infrared spectroscopy as a tool to assess embryo viability: a novel, non-invasive method for embryo selection. Hum Reprod. 2008;23(7):1499–504.
Paulson RJ. Preimplantation genetic screening: what is the clinical efficiency? Fertil Steril. 2017;108(2):228–30.
Barad DH, Darmon SK, Kushnir VA, Albertini DF, Gleicher N. Impact of preimplantation genetic screening on donor oocyte-recipient cycles in the United States. Am J Obstet Gynecol. 2017;217(5):576 e571–8.
Kang HJ, Melnick AP, Stewart JD, Xu K, Rosenwaks Z. Preimplantation genetic screening: who benefits? Fertil Steril. 2016;106(3):597–602.
Qasemi M, Mahdian R, Amidi F. Cell-free DNA discoveries in human reproductive medicine: providing a new tool for biomarker and genetic assays in ART. J Assist Reprod Genet. 2021;38(2):277–88.
Magli MC, Albanese C, Crippa A, Tabanelli C, Ferraretti AP, Gianaroli L: Deoxyribonucleic acid detection in blastocoelic fluid: a new predictor of embryo ploidy and viable pregnancy. Fertil Steril 2019, 111(1):77-85.
Magli MC, Pomante A, Cafueri G, Valerio M, Crippa A, Ferraretti AP, Gianaroli L. Preimplantation genetic testing: polar bodies, blastomeres, trophectoderm cells, or blastocoelic fluid? Fertil Steril. 2016;105(3):676–83 e675.
Tobler KJ, Zhao Y, Ross R, Benner AT, Xu X, Du L, Broman K, Thrift K, Brezina PR, Kearns WG. Blastocoel fluid from differentiated blastocysts harbors embryonic genomic material capable of a whole-genome deoxyribonucleic acid amplification and comprehensive chromosome microarray analysis. Fertil Steril. 2015;104(2):418–25.
Uyar A, Torrealday S, Seli E. Cumulus and granulosa cell markers of oocyte and embryo quality. Fertil Steril. 2013;99(4):979–97.
Pearson H. Safer embryo tests could boost IVF pregnancy rates. Nature. 2006;444(7115):12–3.
Scalici E, Traver S, Molinari N, Mullet T, Monforte M, Vintejoux E, Hamamah S. Cell-free DNA in human follicular fluid as a biomarker of embryo quality. Hum Reprod. 2014;29(12):2661–9.
Xu J, Fang R, Chen L, Chen D, Xiao JP, Yang W, Wang H, Song X, Ma T, Bo S, et al. Noninvasive chromosome screening of human embryos by genome sequencing of embryo culture medium for in vitro fertilization. Proc Natl Acad Sci U S A. 2016;113(42):11907–12.
Belandres D, Shamonki M, Arrach N. Current status of spent embryo media research for preimplantation genetic testing. J Assist Reprod Genet. 2019;36(5):819–26.
Lo YM, Corbetta N, Chamberlain PF, Rai V, Sargent IL, Redman CW, Wainscoat JS. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350(9076):485–7.
Chan KC, Zhang J, Hui AB, Wong N, Lau TK, Leung TN, Lo KW, Huang DW, Lo YM. Size distributions of maternal and fetal DNA in maternal plasma. Clin Chem. 2004;50(1):88–92.
Jiang P, Lo YM. The long and short of circulating cell-free DNA and the ins and outs of molecular diagnostics. Trends in Genetics : TIG. 2016;32(6):360–71.
Yu SC, Lee SW, Jiang P, Leung TY, Chan KC, Chiu RW, Lo YM. High-resolution profiling of fetal DNA clearance from maternal plasma by massively parallel sequencing. Clin Chem. 2013;59(8):1228–37.
Fan HC, Blumenfeld YJ, Chitkara U, Hudgins L, Quake SR. Noninvasive diagnosis of fetal aneuploidy by shotgun sequencing DNA from maternal blood. Proc Natl Acad Sci U S A. 2008;105(42):16266–71.
Shi J, Zhang R, Li J. Size profile of cell-free DNA: a beacon guiding the practice and innovation of clinical testing. Theranostics. 2020;10(11):4737–48.
Yu SC, Chan KC, Zheng YW, Jiang P, Liao GJ, Sun H, Akolekar R, Leung TY, Go AT, van Vugt JM, et al. Size-based molecular diagnostics using plasma DNA for noninvasive prenatal testing. Proc Natl Acad Sci U S A. 2014;111(23):8583–8.
Pan M, Chen P, Lu J, Liu Z, Jia E, Ge Q. The fragmentation patterns of maternal plasma cell-free DNA and its applications in non-invasive prenatal testing. Prenat Diagn. 2020;40(8):911–7.
Qiao L, Yu B, Liang Y, Zhang C, Wu X, Xue Y, Shen C, He Q, Lu J, Xiang J, et al. Sequencing shorter cfDNA fragments improves the fetal DNA fraction in noninvasive prenatal testing. Am J Obstet Gynecol. 2019;221(4):345 e341–11.
Konstantinos S, Petroula T, Evangelos M, Polina G, Argyro G, Sokratis G, Anna R, Andrianos N, Agni P, Michael K, et al. Assessing the practice of LuPOR for poor responders: a prospective study evaluating follicular fluid cfDNA levels during natural IVF cycles. J Assist Reprod Genet. 2020;37(5):1183–94.
Latif Khan H, Bhatti S, Latif Khan Y, Abbas S, Munir Z. Rahman Khan Sherwani IA, Suhail S, Hassan Z, Aydin HH: Cell-free nucleic acids and melatonin levels in human follicular fluid predict embryo quality in patients undergoing in-vitro fertilization treatment. J Gynecol Obstet Hum Reprod. 2020;49(1):101624.
Ho JR, Arrach N, Rhodes-Long K, Ahmady A, Ingles S, Chung K, Bendikson KA, Paulson RJ, McGinnis LK. Pushing the limits of detection: investigation of cell-free DNA for aneuploidy screening in embryos. Fertil Steril. 2018;110(3):467–75 e462.
Liu Y, Shen Q, Zhao X, Zou M, Shao S, Li J, Ren X, Zhang L. Cell-free mitochondrial DNA in human follicular fluid: a promising bio-marker of blastocyst developmental potential in women undergoing assisted reproductive technology. Reproductive Biology and Endocrinology : RB&E. 2019;17(1):54.
Sialakouma A, Karakasiliotis I, Ntala V, Nikolettos N, Asimakopoulos B. Embryonic cell-free DNA (cfDNA) in spent culture medium for aneuploidy screening and its concordance with trophoectoderm biopsy in PGT-A cycles. Hum Reprod. 2021;36(Supplement_1):i377.
Franco J, Carrill, D. Alborno Riaza E, Vill Milla A, Ga Fernande-Vegue R, Soto Borras F, Vega Carrill D, Albornoz A, Martine Acera A, Buen Olalla B, Iniest Perez S, Meli Fullana E et al: Comparative analysis of non-invasive preimplantation genetic testing of aneuploidies (niPGT-A), PGT-A and IVF cycles without aneuploidy testing: preliminary results. Hum Reprod 2021, 36(Supplement_1):i392.
Vera-Rodriguez M, Diez-Juan A, Jimenez-Almazan J, Martinez S, Navarro R, Peinado V, Mercader A, Meseguer M, Blesa D, Moreno I, et al. Origin and composition of cell-free DNA in spent medium from human embryo culture during preimplantation development. Hum Reprod. 2018;33(4):745–56.
Behr B, Pool TB, Milki AA, Moore D, Gebhardt J, Dasig D. Preliminary clinical experience with human blastocyst development in vitro without co-culture. Hum Reprod. 1999;14(2):454–7.
Umetani N, Kim J, Hiramatsu S, Reber HA, Hines OJ, Bilchik AJ, Hoon DS. Increased integrity of free circulating DNA in sera of patients with colorectal or periampullary cancer: direct quantitative PCR for ALU repeats. Clin Chem. 2006;52(6):1062–9.
Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–8.
Regan SLP, Knight PG, Yovich JL, Leung Y, Arfuso F, Dharmarajan A. Granulosa cell apoptosis in the ovarian follicle-a changing view. Front Endocrinol. 2018;9:61.
Lin P, Rui R. Effects of follicular size and FSH on granulosa cell apoptosis and atresia in porcine antral follicles. Mol Reprod Dev. 2010;77(8):670–8.
Best CL, Pudney J, Anderson DJ, Hill JA. Modulation of human granulosa cell steroid production in vitro by tumor necrosis factor alpha: implications of white blood cells in culture. Obstet Gynecol. 1994;84(1):121–7.
Montgomery Rice V, Limback SD, Roby KF, Terranova PF. Differential responses of granulosa cells from small and large follicles to follicle stimulating hormone (FSH) during the menstrual cycle and acyclicity: effects of tumour necrosis factor-alpha. Hum Reprod. 1998;13(5):1285–91.
Acknowledgements
We would like to thank the doctors in the Clinical Center of Reproductive Medicine, State Key Laboratory of Reproductive Medicine in the First Affiliated Hospital involved in the sample and data collection for their support in this study.
Funding
This study was supported by the National Natural Science Foundation of China (61801108) and the Natural Science Foundation of Jiangsu Province (BK20211166, BK20201148).
Author information
Authors and Affiliations
Contributions
Min Pan and Qinyu Ge led the conception and design of the study. Lingbo Cai collected the follicular fluid and SEM samples and clinical data. Min Pan and Huajuan Shi carried out the evaluation of cfDI in samples. Zhiyu Liu carried out the direct qPCR experiments. Min Pan and Qinyu Ge conducted the data analysis, construction of tables and figures, and article revision. Lingbo Cai was involved in study conception and critical revision of the article. All authors approved the final article.
Corresponding authors
Ethics declarations
Ethics approval
Ethics approval was obtained from the Clinical Center of Reproductive Medicine, First Affiliated Hospital, Nanjing in 2020.
Conflict of interest
The authors declare competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pan, M., Shi, H., Liu, Z. et al. The integrity of cfDNA in follicular fluid and spent medium from embryo culture is associated with embryo grade in patients undergoing in vitro fertilization. J Assist Reprod Genet 38, 3113–3124 (2021). https://doi.org/10.1007/s10815-021-02357-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-021-02357-0