Abstract
Purpose
To investigate whether inhibition of LINE-1 affects telomere reprogramming during 2-cell embryo development.
Methods
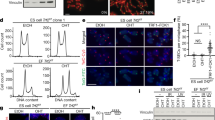
Mouse zygotes were cultured with or without 1 µM azidothymidine (AZT) for up to 15 h (early 2-cell, G1/S) or 24 h (late 2-cell, S/G2). Gene expression and DNA copy number were determined by RT-qPCR and qPCR respectively. Immunostaining and telomeric PNA-FISH were performed for co-localization between telomeres and ZSCAN4 or LINE-1-Orf1p.
Results
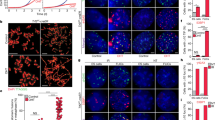
LINE-1 copy number was remarkably reduced in later 2-cell embryos by exposure to 1 µM AZT, and telomere lengths in late 2-cell embryos with AZT were significantly shorter compared to control embryos (P = 0.0002). Additionally, in the absence of LINE-1 inhibition, Dux, Zscan4, and LINE-1 were highly transcribed in early 2-cell embryos, as compared to late 2-cell embryos (P < 0.0001), suggesting that these 2-cell genes are activated at the early 2-cell stage. However, in early 2-cell embryos with AZT treatment, mRNA levels of Dux, Zscan4, and LINE-1 were significantly decreased. Furthermore, both Zscan4 and LINE-1 encoded proteins localized to telomere regions in 2-cell embryos, but this co-localization was dramatically reduced after AZT treatment (P < 0.001).
Conclusions
Upon inhibition of LINE-1 retrotransposition in mouse 2-cell embryos, Dux, Zscan4, and LINE-1 were significantly downregulated, and telomere elongation was blocked. ZSCAN4 foci and their co-localization with telomeres were also significantly decreased, indicating that ZSCAN4 is an essential component of the telomere reprogramming that occurs in mice at the 2-cell stage. Our findings also suggest that LINE-1 may directly contribute to telomere reprogramming in addition to regulating gene expression.



Similar content being viewed by others
References
Waterston, R. H., Lindblad-Toh, K., Birney, E., Rogers, J., Abril, J. F., Agarwal, P., . . . Lander, E. S. (2002). Initial sequencing and comparative analysis of the mouse genome. Nature, 420(6915), 520-562. doi:https://doi.org/10.1038/nature01262
Fu, B., Ma, H., & Liu, D. (2019). Endogenous retroviruses function as gene expression regulatory elements during mammalian pre-implantation embryo development. Int J Mol Sci, 20(3). https://doi.org/10.3390/ijms20030790
Kazazian HH Jr, Moran JV. Mobile DNA in health and disease. N Engl J Med. 2017;377(4):361–70. https://doi.org/10.1056/NEJMra1510092.
Beraldi R, Pittoggi C, Sciamanna I, Mattei E, Spadafora C. Expression of LINE-1 retroposons is essential for murine preimplantation development. Mol Reprod Dev. 2006;73(3):279–87. https://doi.org/10.1002/mrd.20423.
Finnegan DJ. Eukaryotic transposable elements and genome evolution. Trends Genet. 1989;5(4):103–7. https://doi.org/10.1016/0168-9525(89)90039-5.
Ostertag EM, Kazazian HH Jr. Biology of mammalian L1 retrotransposons. Annu Rev Genet. 2001;35:501–38. https://doi.org/10.1146/annurev.genet.35.102401.091032.
Feschotte C, Pritham EJ. DNA transposons and the evolution of eukaryotic genomes. Annu Rev Genet. 2007;41:331–68. https://doi.org/10.1146/annurev.genet.40.110405.090448.
Blackburn EH. Structure and function of telomeres. Nature. 1991;350(6319):569–73. https://doi.org/10.1038/350569a0.
de Lange T. Telomere biology and DNA repair: enemies with benefits. FEBS Lett. 2010;584(17):3673–4. https://doi.org/10.1016/j.febslet.2010.07.030.
Keefe, D. L., Franco, S., Liu, L., Trimarchi, J., Cao, B., Weitzen, S., . . . Blasco, M. A. (2005). Telomere length predicts embryo fragmentation after in vitro fertilization in women--toward a telomere theory of reproductive aging in women. Am J Obstet Gynecol, 192(4), 1256–1260; discussion 1260–1251. doi:https://doi.org/10.1016/j.ajog.2005.01.036
Keefe DL, Liu L, Marquard K. Telomeres and aging-related meiotic dysfunction in women. Cell Mol Life Sci. 2007;64(2):139–43. https://doi.org/10.1007/s00018-006-6466-z.
Keefe DL, Marquard K, Liu L. The telomere theory of reproductive senescence in women. Curr Opin Obstet Gynecol. 2006;18(3):280–5. https://doi.org/10.1097/01.gco.0000193019.05686.49.
Liu L, Blasco M, Trimarchi J, Keefe D. An essential role for functional telomeres in mouse germ cells during fertilization and early development. Dev Biol. 2002;249(1):74–84.
Liu L, Blasco MA, Keefe DL. Requirement of functional telomeres for metaphase chromosome alignments and integrity of meiotic spindles. EMBO Rep. 2002;3(3):230–4. https://doi.org/10.1093/embo-reports/kvf055.
Liu L, Franco S, Spyropoulos B, Moens PB, Blasco MA, Keefe DL. Irregular telomeres impair meiotic synapsis and recombination in mice. Proc Natl Acad Sci U S A. 2004;101(17):6496–501. https://doi.org/10.1073/pnas.0400755101.
Liu, L., Bailey, S. M., Okuka, M., Munoz, P., Li, C., Zhou, L., . . . Keefe, D. L. (2007). Telomere lengthening early in development. Nat Cell Biol, 9(12), 1436-1441. doi:https://doi.org/10.1038/ncb1664
Wright WE, Piatyszek MA, Rainey WE, Byrd W, Shay JW. Telomerase activity in human germline and embryonic tissues and cells. Dev Genet. 1996;18(2):173–9. https://doi.org/10.1002/(sici)1520-6408(1996)18:2%3c173::aid-dvg10%3e3.0.co;2-3.
Henson JD, Neumann AA, Yeager TR, Reddel RR. Alternative lengthening of telomeres in mammalian cells. Oncogene. 2002;21(4):598–610. https://doi.org/10.1038/sj.onc.1205058.
Pickett HA, Reddel RR. Molecular mechanisms of activity and derepression of alternative lengthening of telomeres. Nat Struct Mol Biol. 2015;22(11):875–80. https://doi.org/10.1038/nsmb.3106.
Anifandis G, Messini CI, Dafopoulos K, Messinis IE. Genes and conditions controlling mammalian pre- and post-implantation embryo development. Curr Genomics. 2015;16(1):32–46. https://doi.org/10.2174/1389202916666141224205025.
Hemberger M, Dean W, Reik W. Epigenetic dynamics of stem cells and cell lineage commitment: digging Waddington’s canal. Nat Rev Mol Cell Biol. 2009;10(8):526–37. https://doi.org/10.1038/nrm2727.
Hendrickson, P. G., Dorais, J. A., Grow, E. J., Whiddon, J. L., Lim, J. W., Wike, C. L., . . . Cairns, B. R. (2017). Conserved roles of mouse DUX and human DUX4 in activating cleavage-stage genes and MERVL/HERVL retrotransposons. Nat Genet, 49(6), 925-934. https://doi.org/10.1038/ng.3844
Flach G, Johnson MH, Braude PR, Taylor RA, Bolton VN. The transition from maternal to embryonic control in the 2-cell mouse embryo. Embo j. 1982;1(6):681–6.
Lu X, Sachs F, Ramsay L, Jacques PE, Goke J, Bourque G, Ng HH. The retrovirus HERVH is a long noncoding RNA required for human embryonic stem cell identity. Nat Struct Mol Biol. 2014;21(4):423–5. https://doi.org/10.1038/nsmb.2799.
Macfarlan, T. S., Gifford, W. D., Driscoll, S., Lettieri, K., Rowe, H. M., Bonanomi, D., . . . Pfaff, S. L. (2012). Embryonic stem cell potency fluctuates with endogenous retrovirus activity. Nature, 487(7405), 57-63. doi:https://doi.org/10.1038/nature11244
Sugie K, Funaya S, Kawamura M, Nakamura T, Suzuki MG, Aoki F. Expression of Dux family genes in early preimplantation embryos. Sci Rep. 2020;10(1):19396. https://doi.org/10.1038/s41598-020-76538-9.
Percharde, M., Lin, C. J., Yin, Y., Guan, J., Peixoto, G. A., Bulut-Karslioglu, A., . . . Ramalho-Santos, M. (2018). A LINE1-Nucleolin partnership regulates early development and ESC identity. Cell, 174(2), 391-405.e319. doi:https://doi.org/10.1016/j.cell.2018.05.043
Falco G, Lee SL, Stanghellini I, Bassey UC, Hamatani T, Ko MS. Zscan4: a novel gene expressed exclusively in late 2-cell embryos and embryonic stem cells. Dev Biol. 2007;307(2):539–50. https://doi.org/10.1016/j.ydbio.2007.05.003.
Zalzman, M., Falco, G., Sharova, L. V., Nishiyama, A., Thomas, M., Lee, S. L., . . . Ko, M. S. (2010). Zscan4 regulates telomere elongation and genomic stability in ES cells. Nature, 464(7290), 858-863. doi:https://doi.org/10.1038/nature08882
Macaulay IC, Teng MJ, Haerty W, Kumar P, Ponting CP, Voet T. Separation and parallel sequencing of the genomes and transcriptomes of single cells using G&T-seq. Nat Protoc. 2016;11(11):2081–103. https://doi.org/10.1038/nprot.2016.138.
Okamoto I. Combined immunofluorescence, RNA FISH, and DNA FISH in preimplantation mouse embryos. Methods Mol Biol. 2018;1861:149–59. https://doi.org/10.1007/978-1-4939-8766-5_12.
Kitsou, C., Lazaros, L., Papoudou-Bai, A., Sakaloglou, P., Mastora, E., Lykovardakis, T., . . . Georgiou, I. (2020). Reverse transcriptase affects gametogenesis and preimplantation development in mouse. In Vivo, 34(5), 2269-2276. doi:https://doi.org/10.21873/invivo.12037
Sieh, E., Coluzzi, M. L., Cusella De Angelis, M. G., Mezzogiorno, A., Floridia, M., Canipari, R., . . . Vella, S. (1992). The effects of AZT and DDI on pre- and postimplantation mammalian embryos: an in vivo and in vitro study. AIDS Res Hum Retroviruses, 8(5), 639-649. doi:https://doi.org/10.1089/aid.1992.8.639
Toltzis P, Mourton T, Magnuson T. Effect of zidovudine on preimplantation murine embryos. Antimicrob Agents Chemother. 1993;37(8):1610–3. https://doi.org/10.1128/aac.37.8.1610.
Dai L, Huang Q, Boeke JD. Effect of reverse transcriptase inhibitors on LINE-1 and Ty1 reverse transcriptase activities and on LINE-1 retrotransposition. BMC Biochem. 2011;12:18. https://doi.org/10.1186/1471-2091-12-18.
Jones RB, Garrison KE, Wong JC, Duan EH, Nixon DF, Ostrowski MA. Nucleoside analogue reverse transcriptase inhibitors differentially inhibit human LINE-1 retrotransposition. PLoS ONE. 2008;3(2):e1547. https://doi.org/10.1371/journal.pone.0001547.
Sciamanna I, Vitullo P, Curatolo A, Spadafora C. A reverse transcriptase-dependent mechanism is essential for murine preimplantation development. Genes (Basel). 2011;2(2):360–73. https://doi.org/10.3390/genes2020360.
Jachowicz JW, Bing X, Pontabry J, Boskovic A, Rando OJ, Torres-Padilla ME. LINE-1 activation after fertilization regulates global chromatin accessibility in the early mouse embryo. Nat Genet. 2017;49(10):1502–10. https://doi.org/10.1038/ng.3945.
Kano H, Godoy I, Courtney C, Vetter MR, Gerton GL, Ostertag EM, Kazazian HH Jr. L1 retrotransposition occurs mainly in embryogenesis and creates somatic mosaicism. Genes Dev. 2009;23(11):1303–12. https://doi.org/10.1101/gad.1803909.
Mitsuya, H., Weinhold, K. J., Furman, P. A., St Clair, M. H., Lehrman, S. N., Gallo, R. C., . . . Broder, S. (1985). 3′-Azido-3′-deoxythymidine (BW A509U): an antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc Natl Acad Sci U S A, 82(20), 7096-7100. doi:https://doi.org/10.1073/pnas.82.20.7096
Xie Y, Rosser JM, Thompson TL, Boeke JD, An W. Characterization of L1 retrotransposition with high-throughput dual-luciferase assays. Nucleic Acids Res. 2011;39(3): e16. https://doi.org/10.1093/nar/gkq1076.
Malki S, van der Heijden GW, O’Donnell KA, Martin SL, Bortvin A. A role for retrotransposon LINE-1 in fetal oocyte attrition in mice. Dev Cell. 2014;29(5):521–33. https://doi.org/10.1016/j.devcel.2014.04.027.
Olivero OA. Mechanisms of genotoxicity of nucleoside reverse transcriptase inhibitors. Environ Mol Mutagen. 2007;48(3–4):215–23. https://doi.org/10.1002/em.20195.
Kosebent EG, Uysal F, Ozturk S. Telomere length and telomerase activity during folliculogenesis in mammals. J Reprod Dev. 2018;64(6):477–84. https://doi.org/10.1262/jrd.2018-076.
Ozturk S. Telomerase activity and telomere length in male germ cells. Biol Reprod. 2015;92(2):53. https://doi.org/10.1095/biolreprod.114.124008.
Ozturk S, Sozen B, Demir N. Telomere length and telomerase activity during oocyte maturation and early embryo development in mammalian species. Mol Hum Reprod. 2014;20(1):15–30. https://doi.org/10.1093/molehr/gat055.
Keefe DL. Telomeres, reproductive aging, and genomic instability during early development. Reprod Sci. 2016;23(12):1612–5. https://doi.org/10.1177/1933719116676397.
Nakai-Futatsugi Y, Niwa H. Zscan4 is activated after telomere shortening in mouse embryonic stem cells. Stem Cell Reports. 2016;6(4):483–95. https://doi.org/10.1016/j.stemcr.2016.02.010.
Pardue ML, DeBaryshe PG. Retrotransposons that maintain chromosome ends. Proc Natl Acad Sci U S A. 2011;108(51):20317–24. https://doi.org/10.1073/pnas.1100278108.
Villasante A, Abad JP, Planello R, Mendez-Lago M, Celniker SE, de Pablos B. Drosophila telomeric retrotransposons derived from an ancestral element that was recruited to replace telomerase. Genome Res. 2007;17(12):1909–18. https://doi.org/10.1101/gr.6365107.
Belgnaoui SM, Gosden RG, Semmes OJ, Haoudi A. Human LINE-1 retrotransposon induces DNA damage and apoptosis in cancer cells. Cancer Cell Int. 2006;6:13. https://doi.org/10.1186/1475-2867-6-13.
Gasior SL, Wakeman TP, Xu B, Deininger PL. The human LINE-1 retrotransposon creates DNA double-strand breaks. J Mol Biol. 2006;357(5):1383–93. https://doi.org/10.1016/j.jmb.2006.01.089.
Turner S, Wong HP, Rai J, Hartshorne GM. Telomere lengths in human oocytes, cleavage stage embryos and blastocysts. Mol Hum Reprod. 2010;16(9):685–94. https://doi.org/10.1093/molehr/gaq048.
Tohonen, V., Katayama, S., Vesterlund, L., Jouhilahti, E. M., Sheikhi, M., Madissoon, E., . . . Kere, J. (2015). Novel PRD-like homeodomain transcription factors and retrotransposon elements in early human development. Nat Commun, 6, 8207. doi:https://doi.org/10.1038/ncomms9207
Funding
This study was supported by March of Dimes Grant # 6-FY14-432 and the Stanley H. Kaplan Endowment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, F., Chamani, I.J., Luo, D. et al. Inhibition of LINE-1 retrotransposition represses telomere reprogramming during mouse 2-cell embryo development. J Assist Reprod Genet 38, 3145–3153 (2021). https://doi.org/10.1007/s10815-021-02331-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-021-02331-w