Abstract
Purpose
Nucleoporin 37 (NUP37) has been reported to activate the YAP-TEAD signaling, which is crucial for early embryo development. However, whether NUP37 is involved in oocyte meiosis and embryo development remains largely unknown. The study aimed to clarify the function of Nup37 in oocyte maturation and early embryo development, and to explore the mechanism.
Methods
The expression level and subcellular localization of NUP37 were explored. After knocking down of Nup37 by microinjecting interfering RNA (siRNA), the oocyte maturation rate, aberrant PB1 extrusion rate, and blastocyst formation rate were evaluated. In addition, the effect of the downregulation of Nup37 on YAP-TEAD signaling was confirmed by immunofluorescence staining and real-time quantitative PCR.
Results
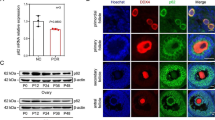
NUP37 was highly expressed in oocytes and early embryos; it mainly localized to the nuclear periphery at mice GV stage oocytes and early embryos. Nup37 depletion led to aberrant PB1 extrusion at the MII stage oocyte and a decreased blastocyst formation rate. The reduction of NUP37 caused YAP1 mislocalization and decreased the expression of Tead1, Tead2, and Tead4 during mice embryo development, thus affecting the YAP-TEAD activity and embryo developmental competence.
Conclusions
In summary, NUP37 played an important role in mice oocyte maturation and preimplantation embryo development.






Similar content being viewed by others
Data availability
The data underlying this article is available in the article and in its online supplementary material.
References
Beck M, Hurt E. The nuclear pore complex: understanding its function through structural insight. Nature reviews Molecular cell biology. 2017;18(2):73–89.
Eibauer M, Pellanda M, Turgay Y, Dubrovsky A, Wild A, Medalia O. Structure and gating of the nuclear pore complex. NAT COMMUN. 2015;6(1).
Schwartz TU. The structure inventory of the nuclear pore complex. J MOL BIOL. 2016;428(10):1986–2000.
Wente SR, Rout MP. The nuclear pore complex and nuclear transport. CSH PERSPECT BIOL. 2010;2(10):a562.
Preston CC, Storm EC, Leonard RJ, Faustino RS. Emerging roles for nucleoporins in reproductive cellular physiology (1). Can J Physiol Pharmacol. 2019;97(4):257–64.
Angelo MAD, Hetzer MW. Structure, dynamics and function of nuclear pore complexes. TRENDS CELL BIOL. 2008;18(10):456–66.
Chen F, Jiao X, Zhang J, Wu D, Ding Z, Wang Y, et al. Nucleoporin35 is a novel microtubule associated protein functioning in oocyte meiotic spindle architecture. EXP CELL RES. 2018;371(2):435–43.
Carvalhal S, Stevense M, Koehler K, Naumann R, Huebner A, Jessberger R, et al. ALADIN is required for the production of fertile mouse oocytes. MOL BIOL CELL. 2017;28(19):2470–8.
Arafah K, Lopez F, Cazin C, Kherraf ZE, Tassistro V, Loundou A, et al. Defect in the nuclear pore membrane glycoprotein 210-like gene is associated with extreme uncondensed sperm nuclear chromatin and male infertility: a case report. HUM REPROD. 2021;36(3):693–701.
Smitherman M, Lee K, Swanger J, Kapur R, Clurman BE. Characterization and targeted disruption of murine Nup50, a p27(Kip1)-interacting component of the nuclear pore complex. MOL CELL BIOL. 2000;20(15):5631–42.
Okita K, Kiyonari H, Nobuhisa I, Kimura N, Aizawa S, Taga T. Targeted disruption of the mouse ELYS gene results in embryonic death at peri-implantation development. GENES CELLS. 2004;9(11):1083–91.
Wu X, Kasper LH, Mantcheva RT, Mantchev GT, Springett MJ, van Deursen JM. Disruption of the FG nucleoporin NUP98 causes selective changes in nuclear pore complex stoichiometry and function. Proc Natl Acad Sci U S A. 2001;98(6):3191–6.
Yan L, Yang M, Guo H, Yang L, Wu J, Li R, et al. Single-cell RNA-Seq profiling of human preimplantation embryos and embryonic stem cells. NAT STRUCT MOL BIOL. 2013;20(9):1131–9.
Asakawa H, Kojidani T, Yang HJ, Ohtsuki C, Osakada H, Matsuda A, et al. Asymmetrical localization of Nup107–160 subcomplex components within the nuclear pore complex in fission yeast. PLOS GENET. 2019;15(6):e1008061.
Duan X, Sun SC. Actin cytoskeleton dynamics in mammalian oocyte meiosis. BIOL REPROD. 2019;100(1):15–24.
di Pietro F, Echard A, Morin X. Regulation of mitotic spindle orientation: an integrated view. EMBO REP. 2016;17(8):1106–30.
Babariya D, Fragouli E, Alfarawati S, Spath K, Wells D. The incidence and origin of segmental aneuploidy in human oocytes and preimplantation embryos. HUM REPROD. 2017;32(12):2549–60.
Mishra RK, Chakraborty P, Arnaoutov A, Fontoura BMA, Dasso M. The Nup107-160 complex and γ-TuRC regulate microtubule polymerization at kinetochores. NAT CELL BIOL. 2010;12(2):164–9.
Luo X, Liu Y, Feng W, Lei L, Du Y, Wu J, et al. NUP37, a positive regulator of YAP/TEAD signaling, promotes the progression of hepatocellular carcinoma. Oncotarget. 2017;8(58):98004–13.
Varelas X. The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. DEVELOPMENT. 2014;141(8):1614–26.
Pocaterra A, Romani P, Dupont S. YAP/TAZ functions and their regulation at a glance. J CELL SCI. 2020133(2).
Kaneko KJ, Cullinan EB, Latham KE, DePamphilis ML. Transcription factor mTEAD-2 is selectively expressed at the beginning of zygotic gene expression in the mouse. DEVELOPMENT. 1997;124(10):1963–73.
Yagi R, Kohn MJ, Karavanova I, Kaneko KJ, Vullhorst D, DePamphilis ML, et al. Transcription factor TEAD4 specifies the trophectoderm lineage at the beginning of mammalian development. DEVELOPMENT. 2007;134(21):3827–36.
Nishioka N, Yamamoto S, Kiyonari H, Sato H, Sawada A, Ota M, et al. Tead4 is required for specification of trophectoderm in pre-implantation mouse embryos. MECH DEVELOP. 2008;125(3–4):270–83.
Nishioka N, Inoue K, Adachi K, Kiyonari H, Ota M, Ralston A, et al. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. DEV CELL. 2009;16(3):398–410.
Sha QQ, Zheng W, Wu YW, Li S, Guo L, Zhang S, et al. Dynamics and clinical relevance of maternal mRNA clearance during the oocyte-to-embryo transition in humans. NAT COMMUN. 2020;11(1):4917.
Yu C, Ji SY, Dang YJ, Sha QQ, Yuan YF, Zhou JJ, et al. Oocyte-expressed yes-associated protein is a key activator of the early zygotic genome in mouse. CELL RES. 2016;26(3):275–87.
Sawada A, Kiyonari H, Ukita K, Nishioka N, Imuta Y, Sasaki H. Redundant roles of Tead1 and Tead2 in notochord development and the regulation of cell proliferation and survival. MOL CELL BIOL. 2008;28(10):3177–89.
Gerri C, McCarthy A, Alanis-Lobato G, Demtschenko A, Bruneau A, Loubersac S, et al. Initiation of a conserved trophectoderm program in human, cow and mouse embryos. NATURE. 2020;587(7834):443–7.
Hashimoto M, Sasaki H. Epiblast formation by TEAD-YAP-dependent expression of pluripotency factors and competitive elimination of unspecified cells. DEV CELL. 2019;50(2):139–54.
Gamini R, Han W, Stone JE, Schulten K. Assembly of Nsp1 nucleoporins provides insight into nuclear pore complex gating. PLOS COMPUT BIOL. 2014;10(3):e1003488.
Kapinos LE, Huang B, Rencurel C, Lim RYH. Karyopherins regulate nuclear pore complex barrier and transport function. J CELL BIOL. 2017;216(11):3609–24.
Tamm C, Bower N, Anneren C. Regulation of mouse embryonic stem cell self-renewal by a Yes-YAP-TEAD2 signaling pathway downstream of LIF. J CELL SCI. 2011;124(Pt 7):1136–44.
Landin-Malt A, Benhaddou A, Zider A, Flagiello D. An evolutionary, structural and functional overview of the mammalian TEAD1 and TEAD2 transcription factors. GENE. 2016;591(1):292–303.
Xu Y, Shi Y, Fu J, Yu M, Feng R, Sang Q, et al. Mutations in PADI6 cause female infertility characterized by early embryonic arrest. The American Journal of Human Genetics. 2016;99(3):744–52.
Yang P, Yin C, Li M, Ma S, Cao Y, Zhang C, et al. Mutation analysis of tubulin beta 8 classVIII in infertile females with oocyte or embryonic defects. CLIN GENET. 2021;99(1):208–14.
Mu J, Wang W, Chen B, Wu L, Li B, Mao X, et al. Mutations in NLRP2 and NLRP5 cause female infertility characterised by early embryonic arrest. J MED GENET. 2019;56(7):471–80.
Feng R, Sang Q, Kuang Y, Sun X, Yan Z, Zhang S, et al. Mutations in TUBB8 and human oocyte meiotic arrest. N Engl J Med. 2016;374(3):223–32.
Acknowledgements
The authors would like to thank Jiaxi Cheng for her careful discussion on the content of the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (2017YFA0103801) and the National Natural Science Foundation of China (No. 31871447, No. 31571544, and No. 31522034).
Author information
Authors and Affiliations
Contributions
Q.G., Q.L., N.W., and A.S. performed the experiments. Q.G. and J.W. analyzed the data. Q.G., Q.L., and L.Y. designed the study. Q.G., Q.L, and L.Y. wrote the manuscript with help from all authors. The manuscript has been approved by all authors for publication.
Corresponding author
Ethics declarations
Ethics approval
Animal experimental procedures were followed as the Institutional Animal Welfare and Ethics Committee policies of Peking University (LA2018261).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information

ESM 1
Subcellular localization of Nup37 at the 8-cell stage. Immunofluorescent staining of Nup37 showing that in some cases Nup37 localized at the nucleus at the 8-cell stage. Scale bar, 20μm. (PNG 3604 kb)

ESM 2
NUP37 inhibition did not affect chromosome ploidy. (A) Metaphase spread of chromosomes from SiNC and SiNup37 MII oocytes, chromosomes were stained with DAPI. Scale bar, 10μm. (B) The chromosome ploidy was recorded and compared between the SiNC (n = 24) and SiNup37 (n = 46) group. (C) There was no significant difference in euploidy rate between SiNC and SiNup37 (P = 0.603, Chi-Squared Test). (PNG 561 kb)
Rights and permissions
About this article
Cite this article
Guo, Q., Liu, Q., Wang, N. et al. The function of Nucleoporin 37 on mouse oocyte maturation and preimplantation embryo development. J Assist Reprod Genet 39, 107–116 (2022). https://doi.org/10.1007/s10815-021-02330-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-021-02330-x