Abstract
Purpose
To assess the effect of high ovarian response on oocyte quality and ovarian stimulation cycle outcomes.
Methods
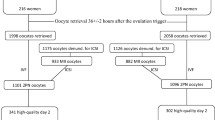
A retrospective cohort study conducted at three IVF units. The high ovarian response (HOR) and polycystic ovary syndrome (PCOS) with HOR (PCOS HOR) groups included 151 and 13 women who underwent controlled ovarian stimulation (COS) resulting in more than 15 retrieved oocytes, for a total of 1863 and 116 cultured embryos, respectively. The normal ovarian response (NOR) group comprised 741 women with 6–15 retrieved oocytes, resulting in 4907 cultured embryos. Data collected included fresh cycle data and pregnancy rates, in addition to annotation of morphokinetic events from time of pronuclei fading to time of initiation of blastocyst formation of embryos cultured in a time lapse incubator, including occurrence of direct unequal cleavage at first cleavage (DUC-1) (less than 5 h from two to three blastomeres). Comparison was made between morphokinetic parameters between the 3 groups. Cycle outcomes were compared in the high vs. normal ovarian response groups.
Results
Oocyte maturation rate was significantly lower in the HOR vs. NOR groups (56.5% vs. 90.0%, p < 0.001), while the fertilization rates were similar (60.2% vs. 58.1%, p = 0.397). The prevalence of DUC-1 embryos was higher in the PCOS HOR and the HOR groups as compared to the NOR group (22.7% vs. 16.2% and 12.0%, respectively, p < 0.001). After exclusion of DUC-1 embryos, remaining embryos from the NOR and HOR groups reached the morphokinetic milestones at similar rates, with comparable implantation and clinical pregnancy rates, while the PCOS HOR showed shorter time to 5 blastomeres compared to the NOR and HOR groups.
Conclusions
High ovarian response might be associated with decreased oocyte quality, manifested as a higher proportion of immature oocytes and higher rate of direct uneven cleavage embryos, while embryos exhibiting normal first cleavage have similar temporal milestones and implantation potential.

Similar content being viewed by others
Data availability
Available upon request.
References
Kosmas IP, Kolibianakis EM, Devroey P. Association of estradiol levels on the day of hCG administration and pregnancy achievement in IVF: a systematic review. Hum Reprod. 2004;19:2446–53.
Venetis CA, Kolibianakis EM, Bosdou JK, Tarlatzis BC. Progesterone elevation and probability of pregnancy after IVF: a systematic review and meta-analysis of over 60 000 cycles. Hum Reprod Update. 2013;19:433–57.
Valbuena D, Jasper M, Remohí J, Pellicer A, Simón C. Ovarian stimulation and endometrial receptivity. Hum Reprod. 1999;14(Suppl 2):107–11.
Valbuena D, Martin J, de Pablo JL, Remohí J, Pellicer A, Simón C. Increasing levels of estradiol are deleterious to embryonic implantation because they directly affect the embryo. Fertil Steril. 2001;76:962–8.
Kok JD, Looman CW, Weima SM, te Velde ER. A high number of oocytes obtained after ovarian hyperstimulation for in vitro fertilization or intracytoplasmic sperm injection is not associated with decreased pregnancy outcome. Fertil Steril. 2006;85:918–24.
Arce JC, Andersen AN, Fernández-Sánchez M, Visnova H, Bosch E, García-Velasco JA, Barri P, de Sutter P, Klein BM, Fauser BC. Ovarian response to recombinant human follicle-stimulating hormone: a randomized, antimullerian hormone-stratified, dose-response trial in women undergoing in vitro fertilization/intracytoplasmic sperm injection. Fertil Steril. 2014;102:1633-40.e5.
Motato Y, de los Santos MJ, Escriba MJ, Ruiz BA, Remohí J, Meseguer M. Morphokinetic analysis and embryonic prediction for blastocyst formation through an integrated time-lapse system. Fertil Steril. 2016;105:376-84.e9.
Kirkegaard K, Kesmodel US, Hindkjær JJ, Ingerslev HJ. Time-lapse parameters as predictors of blastocyst development and pregnancy outcome in embryos from good prognosis patients: a prospective cohort study. Hum Reprod. 2013;28:2643–51.
Meseguer M, Herrero J, Tejera A, Hilligsøe KM, Ramsing NB, Remohí J. The use of morphokinetics as a predictor of embryo implantation. Hum Reprod. 2011;26:2658–71.
Rubio I, Kuhlmann R, Agerholm I, Kirk J, Herrero J, Escriba MJ, et al. Limited implantation success of direct-cleaved human zygotes: a time-lapse study. Fertil Steril. 2012;98:1458–63.
Zhan Q, Ye Z, Clarke R, Rosenwaks Z, Zaninovic N. Direct Unequal cleavages: embryo developmental competence, genetic constitution and clinical outcome. PLoS One. 2016;11:e0166398.
Ciray HN, Campbell A, Agerholm IE, Aguilar J, Chamayou S, Esbert M, et al. Time-Lapse User Group. Proposed guidelines on the nomenclature and annotation of dynamic human embryo monitoring by a time-lapse user group. Hum Reprod. 2014;29:2650–60.
Simón C, Cano F, Valbuena D, Remohí J, Pellicer A. Clinical evidence for a detrimental effect on uterine receptivity of high serum oestradiol concentrations in high and normal responder patients. Hum Reprod. 1995;10:2432–7.
Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41–7.
Athayde Wirka K, Chen AA, Conaghan J, Ivani K, Gvakharia M, Behr B, et al. Atypical embryo phenotypes identified by time-lapse microscopy: high prevalence and association with embryo development. Fertil Steril. 2014;101:1637–48.
Van Blerkom J, Davis P. Differential effects of repeated ovarian stimulation on cytoplasmic and spindle organization in metaphase II mouse oocytes matured in vivo and in vitro. Hum Reprod. 2001;16:757–64.
Fauser BC, Devroey P. Reproductive biology and IVF: ovarian stimulation and luteal phase consequences. Trends Endocrinol Metab. 2003;14:236–42.
Wood JR, Dumesic DA, Abbott DH, Strauss JF. Molecular abnormalities in oocytes from women with polycystic ovary syndrome revealed by microarray analysis. J Clin Endocrinol Metab. 2007;92:705–13.
Funding
Israel Innovation Authority – Kamin grant 55326.
Author information
Authors and Affiliations
Contributions
Natali Schachter-Safrai and Gilad Karavani have contributed substantially to the conception and design of the study, analysis and interpretation of data, and drafting the article. Efrat Esh-Broder and Eliahu Levitas have contributed substantially to the acquisition, analysis and interpretation of data, and revision of the article. Tamar Wainstock has contributed substantially to the conception and design of the study, analysis and interpretation of data, and revision of the article. Iris Har-Vardi and Assaf Ben-Meir have contributed substantially to the conception and design of the study, acquisition, analysis and interpretation of data, and drafting and revision of the article. All authors have approved the final version of the study.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Natali Schachter-Safrai and Gilad Karavani should be considered as equal contribution first authors.
Iris Har-Vardi and Assaf Ben-Meir should be considered as equal contribution last authors.
Rights and permissions
About this article
Cite this article
Schachter-Safrai, N., Karavani, G., Esh-Broder, E. et al. High ovarian response to ovarian stimulation: effect on morphokinetic milestones and cycle outcomes. J Assist Reprod Genet 38, 3083–3090 (2021). https://doi.org/10.1007/s10815-021-02323-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-021-02323-w