Abstract
Purpose
Oxygen tension during the in vitro maturation (IVM) of oocytes is important for oocyte developmental competence. A conflict exists in the literature as to whether low oxygen during IVM is detrimental or beneficial to the oocyte. Many research and clinical labs use higher than physiological oxygen tension perhaps believing that low-oxygen tension is detrimental to oocyte development. Other studies show that glucose is important if low-oxygen tension is used during maturation. In this study, we look at the link between low oxygen and glucose availability during IVM to resolve misconceptions around low-oxygen tension during IVM.
Methods
Bovine cumulus oocyte complexes (COCs) were matured at 20% vs 7% oxygen in media containing differing glucose concentrations or varying availability. Cleavage and blastocyst rates were recorded. RT-PCR determined expression levels of metabolic, oxygen, and stress-responsive genes following IVM.
Results
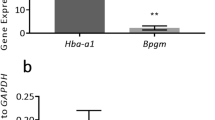
Embryo development in 7% oxygen groups with 2.3mM glucose/low glucose availability was lower than 20% oxygen groups. Under 7% oxygen with 5.6mM glucose or higher glucose availability, rates were restored to those seen in 20% oxygen. Expressions of BNIP3, ENO1, GAPDH, and SLC2A1, were upregulated in 7% oxygen/low glucose, compared to 20% oxygen groups. BNIP3 expression was higher in 7% oxygen group with low glucose availability compared to the 20% groups.
Conclusion
Oocyte developmental competence is negatively impacted following IVM in low oxygen when glucose availability is limited. Glucose concentration and physical culture conditions need to be considered when comparing the effects of different oxygen concentrations during IVM.







Similar content being viewed by others
References
Child TJ, Abdul-Jalil AK, Gulekli B, Tan SL. In vitro maturation and fertilization of oocytes from unstimulated normal ovaries, polycystic ovaries, and women with polycystic ovary syndrome. Fertil Steril. 2001;76(5):936–42.
Kumar P, Sait SF, Sharma A, Kumar M. Ovarian hyperstimulation syndrome. J Hum Reprod Sci. 2011;4(2):70–5.
Vuong LN, Ho VNA, Ho TM, Dang VQ, Phung TH, Giang NH, Le AH, Pham TD, Wang R, Norman RJ, Smitz J, Gilchrist RB, Mol BW. Effectiveness and safety of in vitro maturation of oocytes versus in vitro fertilisation in women with high antral follicle count: study protocol for a randomised controlled trial. BMJ Open. 2018;8(12):e023413-e023413.
Maman E, Meirow D, Brengauz M, Raanani H, Dor J, Hourvitz A. Luteal phase oocyte retrieval and in vitro maturation is an optional procedure for urgent fertility preservation. Fertil Steril. 2011;95(1):64–7.
Ellenbogen A, Shavit T, Shalom-Paz E. IVM results are comparable and may have advantages over standard IVF. Facts, views & vision in ObGyn. 2014;6(2):77–80.
Wu B, Zan L, Quan F, Wang H. A Novel Discipline in Embryology — Animal Embryo Breeding, in New Discoveries in Embryology. In: Wu B, editor. IntechOpen: Open Access; 2015.
Lonergan P, Fair T. Maturtion of oocytes in vitro. Anim Rev Annu Biosci. 2016;4(1):255–68.
Rizos D, Ward F, Duffy P, Boland MP, Lonergan P. Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: implications for blastocyst yield and blastocyst quality. Mol Reprod Dev. 61(2):234–48. https://doi.org/10.1002/mrd.1153.
Ealy A, Wooldridge LK, SR MC. Post-transfer consequences of in vitro-produced embryos in cattle. Anim Sci J, 2019. 97(6):2568. https://doi.org/10.1093/jas/skz116.
Lonergan P, Rizos D, Gutierrez-Adan A, Fair T, Boland MP. Oocyte and Embryo Quality: Effect of Origin, Culture Conditions and Gene Expression Patterns. Reprod Domest Anim. 2003;38(4):259–67.
Niemann H, Wrenzycki C. Alterations of expression of developmentally important genes in preimplantation bovine embryos by in vitro culture conditions: Implications for subsequent development. Theriogenology. 2000;53(1):21–34.
Fischer B, Bavister BD. Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. Reproduction (Cambridge, England). 1993;99(2):673–9.
Mastroianni L, Jones R. Oxygen tension within the rabbit fallopian tube. Reproduction (Cambridge, England). 1965;9(1):99–102.
Van Blerkom J. Epigenetic influences on oocyte developmental competence: perifollicular vascularity and intrafollicular oxygen. J Assist Reprod Genet. 1998;15(5):226–34.
Huey S, Abuhamad A, Barroso G, Kolm P, Mayer J, Oehninger S. Perifollicular blood flow doppler indices, but not follicular pO2, pCO2, or pH, predict oocyte developmental competence in in vitro fertilization. Fertil Steril. 1999;72(4):707–12.
Tatemoto H, Muto N, Sunagawa I, Shinjo A, Nakata T. Protection of porcine oocytes against cell damage caused by oxidative stress during in vitro maturation: role of superoxide dismutase activity in porcine follicular fluid. Biol Reprod. 2004;71(4):1150–7.
Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003;79(4):829–43.
Agarwal A, Prabakaran S, Allamaneni SSSR. Relationship between oxidative stress, varicocele and infertility: a meta-analysis. Reprod BioMed Online. 2006;12(5):630–3.
Agarwal A, Gupta S, Sikka S. The role of free radicals and antioxidants in reproduction. Curr Opin Obstet Gynecol. 2006;18(3):325–32.
Hashimoto S, Minami N, Takakura R, Yamada M, Imai H, Kashima N. Low oxygen tension during in vitro maturation is beneficial for supporting the subsequent development of bovine cumulus–oocyte complexes. Mol Reprod Dev. 2000;57(4):353–60.
Johnson MH, Nasresfahani MH. Radical solutions and cultural problems: Could free oxygen radicals be responsible for the impaired development of preimplantation mammalian embryos in vitro? BioEssays. 1994;16(1):31–8.
Alonso-Alvarez C, Bertrand S, Devevey G, Prost J, Faivre B, Sorci G. Increased susceptibility to oxidative stress as a proximate cost of reproduction. Ecol Lett. 2004;7(5):363–8.
Rinaudo PF, et al. Effects of oxygen tension on gene expression in preimplantation mouse embryos. Fertil Steril. 2006;86(4):1265.e1–1265.e36.
Sutton-McDowall M, Gilchrist R, Thompson J. Glucosamine supplementation during in vitro maturation leads to perturbed developmental capacity of bovine cumulus oocyte complexes. Reprod Fertil Dev. 2005;17(2):300.
Leese HJ, Lenton EA. Glucose and lactate in human follicular fluid: concentrations and interrelationships. Hum Reprod (Oxford). 1990;5(8):915–9.
Johnson AE, Lane M, Gardiner DK, Diekman MA, Krisher RL. Changes in follicular fluid environment between 5mm and 10mm follicles. Annual Conference of the Society for the Study of Reproduction, 2001.
Hashimoto S. Application of In Vitro Maturation to Assisted Reproductive Technology. J Reprod Dev. 2009;55(1):1–10.
Cetica P, Pintos L, Dalvit G, Beconi M. Activity of key enzymes involved in glucose and triglyceride catabolism during bovine oocyte maturation in vitro. Reproduction (Cambridge, England). 2002;124(5):675–81.
Biggers JD, Whittingham DG, Donahue RP. The pattern of energy metabolism in the mouse oocyte and zygote. PNAS. 1967;58(2):560–7.
Rieger D, Loskutoff NM. Changes in the metabolism of glucose, pyruvate, glutamine and glycine during maturation of cattle oocytes in vitro. J Reprod Fertil. 1994;100(1):257–62.
Saito T, Hiroi M, Kato T. Development of glucose utilization studied in single oocytes and preimplantation embryos from mice. Biol Reprod. 1994;50(2):266–70.
Dan-Goor M, Sasson S, Davarashvili A, Almagor M. Expression of glucose transporter and glucose uptake in human oocytes and preimplantation embryos. Hum Reprod (Oxford). 1997;12(11):2508–10.
Khurana NK. and. Niemann H. Effects of oocyte quality, oxygen tension, embryo density, cumulus cells and energy substrates on cleavage and morula/blastocyst formation of bovine embryos. Theriogenology. 2000;54(5):741–56.
Clark AR, Stokes YM, Lane M, Thompson JG. Mathematical modelling of oxygen concentration in bovine and murine cumulus–oocyte complexes. Reproduction (Cambridge, England). 2006;131(6):999–1006.
Thompson JG, Brown HM, Kind KL, Russell DL. The Ovarian Antral Follicle: Living on the Edge of Hypoxia or Not? Biol Reprod. 2015;92(6):153-153.
Downs SM, Utecht AM. Metabolism of radiolabeled glucose by mouse oocytes and oocyte-cumulus cell complexes. Biol Reprod. 1999;60(6):1446–52.
Spindler RE, Pukazhenthi BS, Wildt DE. Oocyte metabolism predicts the development of cat embryos to blastocyst in vitro. Mol Reprod Dev. 2000;56(2):163–71.
Sutton-McDowall ML, Gilchrist RB, Thompson JG. The pivotal role of glucose metabolism in determining oocyte developmental competence. Reproduction (Cambridge, England). 2010;139(4):685–95.
Xie H-L, Wang YB, Jiao GZ, Kong DL, Li Q, Li H, Zheng LL, Tan JH. Effects of glucose metabolism during in vitro maturation on cytoplasmic maturation of mouse oocytes. Sci Rep. 2016;6(1):20764-20764.
Downs SM, Hudson ED. Energy substrates and the completion of spontaneous meiotic maturation. Zygote (Cambridge). 2000;8(4):339–51.
Urner F, Sakkas D. Influence of glucose on protein tyrosine phosphorylation in mouse spermatozoa. Hum Reprod (Oxford). 1999;14(Suppl_3):13-13.
Bermejo-Álvarez P, Lonergan P, Rizos D, Guttierez-Adan A. Low oxygen tension during IVM improves bovine oocyte competence and enhances anaerobic glycolysis. Reprod BioMed Online. 2009;20(3):341–9.
Sutton-McDowell ML, Gilchrist RB, Thompson JG. Cumulus expansion and glucose utilisation by bovine cumulus–oocyte complexes during in vitro maturation: the influence of glucosamine and follicle-stimulating hormone. Reproduction. 2004;128(3):313–9.
Dunning KR, Watson LN, Sharkey DJ, Brown HM, Norman RJ, Thompson JG, Robker RL, Russell DL. Molecular filtration properties of the mouse expanded cumulus matrix: controlled supply of metabolites and extracellular signals to cumulus cells and the oocyte. Biol Reprod. 2012;87(4):89-89.
Tesarík J. Comparison of acrosome reaction-inducing activities of human cumulus oophorus, follicular fluid and ionophore A23187 in human sperm populations of proven fertilizing ability in vitro. J Reprod Fertil. 1985;74(2):383–8.
Hong S-J, Chiu PC-N, Lee K-F, Tse JY-M, Ho P-C, Yueng WS-B. Cumulus cells and their extracellular matrix affect the quality of the spermatozoa penetrating the cumulus mass. Fertil Steril. 2009;92(3):971–8.
Carrell DT, Middleton RG, Peterson CM, Jones KP, Urry RL. Role of the cumulus in the selection of morphologically normal sperm and induction of the acrosome reaction during human in vitro fertilization. Arch Androl. 1993;31(2):133–7.
Haidri AA, Miller IM, Gwatkin RB. Culture of mouse oocytes in vitro, using a system without oil or protein. J Reprod Fertil. 1971;26(3):409–11.
Gwatkin RB, Haidri AA. Oxygen requirements for the maturation of hamster oocytes. J Reprod Fertil. 1974;37(1):127–9.
Banwell KM, Lane M, Russell DL, Kind KL, Thompson JG. Oxygen concentration during mouse oocyte in vitro maturation affects embryo and fetal development. Hum Reprod (Oxford). 2007;22(10):2768–75.
Preis KA, Seidel GE, Gardner DK. Reduced oxygen concentration improves the developmental competence of mouse oocytes following in vitro maturation. Mol Reprod Dev. 2007;74(7):893–903.
Pinyopummintr T, Bavister BD. Optimum gas atmosphere for in vitro maturation and in vitro fertilization of bovine oocytes. Theriogenology. 1995;44(4):471–7.
de Castro e Paula LA, Hansen PJ. Interactions between oxygen tension and glucose concentration that modulate actions of heat shock on bovine oocytes during in vitro maturation. Theriogenology. 2007;68(5):763–70.
Watson AJ, de Sousa P, Caveney A, Barcroft LC, Natale D, Urquhart J, et al. Impact of bovine oocyte maturation media on oocyte transcript levels, blastocyst development, cell number, and apoptosis. Biol Reprod. 2000;62(2):355–64.
Fagbohun CF, Down SM. Maturation of the mouse oocyte-cumulus cell complex: stimulation by lectins. Biol Reprod. 1990;42(3):413–23.
Vanderhyden BC, Caron PJ, Buccione R, Eppig JJ. Developmental pattern of the secretion of cumulus expansion-enabling factor by mouse oocytes and the role of oocytes in promoting granulosa cell differentiation. Dev Biol. 1990;140(2):307–17.
Society, I.E.T.S, Manual of the International Embryo Transfer Society: A procedural guide and general information for the use of embryo transfer technology, emphasizing sanitary precautions. 1998, The Society, 1998.
Kind KL, Tam KKY, Banwell KM, Gauld AD, Russell DL, Macpherson AM, et al. Oxygen-regulated gene expression in murine cumulus cells. Reprod Fertil Dev. 2015;27(2):407–18.
Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell (Cambridge). 2012;148(3):399–408.
Bashan N, Burdett E, Hundal HS, Klip A. Regulation of glucose transport and GLUT1 glucose transporter expression by O2 in muscle cells in culture. Am J Phys Cell Phys. 1992;262(3):682–90.
Ebert BL, Firth JD, Ratcliffe PJ. Hypoxia and Mitochondrial Inhibitors Regulate Expression of Glucose Transporter-1 via Distinct Cis-acting Sequences. J Biol Chem. 1995;270(49):29083–9.
Baddela VS, Sharma A, Michaelis M, Vanselow J. HIF1 driven transcriptional activity regulates steroidogenesis and proliferation of bovine granulosa cells. Sci Rep. 2020; 10(1):3906-3906.
Kind KL, Banwell KM, Gebhardt KM, Macpherson A, Gauld A, Russell DL, et al. Microarray analysis of mRNA from cumulus cells following in vivo or in vitro maturation of mouse cumulus-oocyte complexes. Reprod Fertil Dev. 2013;25(2):426–38.
Thompson J, Lane M, Gilchrist R. Metabolism of the bovine cumulus-oocyte complex and influence on subsequent developmental competence. Society of Reproduction and Fertility supplement. 2007;64:179–90.
Chen L, Russell PT, Larsen WJ. Functional significance of cumulus expansion in the mouse: roles for the preovulatory synthesis of hyaluronic acid within the cumulus mass. Mol Reprod Dev. 1993;34:87–93.
Tirone E, Siracusa G, Hascall VC, Frajese G, Sulustri A. Oocytes preserve the ability of mouse cumulus cells in culture to synthesize hyaluronic acid and dermatan sulfate. Dev Biol. 1993;160(2):405–12.
Downs SM, Humpherson PG, Leese HJ. Meiotic induction in cumulus cell-enclosed mouse oocytes: involvement of the pentose phosphate pathway. Biol Reprod. 1998;58(4):1084–94.
Gardner DK. Lactate production by the mammalian blastocyst: Manipulating the microenvironment for uterine implantation and invasion? BioEssays. 2015;37(4):364–71.
Dumollard R, Ward Z, Carroll J, Duchen MR. Regulation of redox metabolism in the mouse oocyte and embryo. Development (Cambridge). 2006;134(3):455–65.
Redmann M, Darley-Usmar V, Zhang J. The role of autophagy, mitophagy and lysosomal functions in modulating bioenergetics and survival in the context of redox and proteotoxic damage: implications for neurodegenerative diseases. Aging Dis. 2016;7(2):150–62.
Redmann M, Dodson M, Boyer-Guittaut M, Darley-Usmar V, Zhang J. Mitophagy mechanisms and role in human diseases. Int J Biochem Cell Biol. 2014;53:127–33.
Quinsay MN, Lee Y, Rikka S, Sayen RM, Molkentin JD, Gottleib RA, et al. Bnip3 mediates permeabilization of mitochondria and release of cytochrome c via a novel mechanism. J Mol Cell Cardiol. 2009;48(6):1146–56.
Thompson JGE, Simpson AC, Pugh PA, Donnelly PE, Tervit HR. Effect of oxygen concentration on in-vitro development of preimplantation sheep and cattle embryos. J Reprod Fertil. 1990;89(2):573–8.
Stiehl DP, Jelkmann W, Wenger RH, Hellwig-Burgel T. Normoxic induction of the hypoxia-inducible factor 1α by insulin and interleukin-1β involves the phosphatidylinositol 3-kinase pathway. FEBS Lett. 2002;512(1):157–62.
Turhan A, Pereira MT, Schuler G, Bleul U, Kowalewski MP. Hypoxia-inducible factor (HIF1alpha) inhibition modulates cumulus cell function and affects bovine oocyte maturation in vitro. Biol Reprod. 2020.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Whitty, A., Kind, K.L., Dunning, K.R. et al. Effect of oxygen and glucose availability during in vitro maturation of bovine oocytes on development and gene expression. J Assist Reprod Genet 38, 1349–1362 (2021). https://doi.org/10.1007/s10815-021-02218-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-021-02218-w