Abstract
Purpose
To assess whether utilization of a mathematical ranking algorithm for assistance with embryo selection improves clinical outcomes compared with traditional embryo selection via morphologic grading in single vitrified warmed euploid embryo transfers (euploid SETs).
Methods
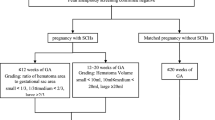
A retrospective cohort study in a single, academic center from September 2016 to February 2020 was performed. A total of 4320 euploid SETs met inclusion criteria and were included in the study. Controls included all euploid SETs in which embryo selection was performed by a senior embryologist based on modified Gardner grading (traditional approach). Cases included euploid SETs in which embryo selection was performed using an automated algorithm-based approach (algorithm-based approach). Our primary outcome was implantation rate. Secondary outcomes included ongoing pregnancy/live birth rate and clinical loss rate.
Results
The implantation rate and ongoing pregnancy/live birth rate were significantly higher when using the algorithm-based approach compared with the traditional approach (65.3% vs 57.8%, p<0.0001 and 54.7% vs 48.1%, p=0.0001, respectively). After adjusting for potential confounding variables, utilization of the algorithm remained significantly associated with improved odds of implantation (aOR 1.51, 95% CI 1.04, 2.18, p=0.03) ongoing pregnancy/live birth (aOR 1.99, 95% CI 1.38, 2.86, p=0.0002), and decreased odds of clinical loss (aOR 0.42, 95% CI 0.21, 0.84, p=0.01).
Conclusions
Clinical implementation of an automated mathematical algorithm for embryo ranking and selection is significantly associated with improved implantation and ongoing pregnancy/live birth as compared with traditional embryo selection in euploid SETs.

Similar content being viewed by others
Data Availability
The data underlying this article may be obtained upon reasonable request.
References
Nazem TG, Sekhon L, Lee JA, Overbey J, Pan S, Duke M, et al. The correlation between morphology and implantation of euploid human blastocysts. Reprod BioMed Online. 2019;38(2):169–76.
Irani M, Reichman D, Robles A, Melnick A, Davis O, Zaninovic N, et al. Morphologic grading of euploid blastocysts influences implantation and ongoing pregnancy rates. Fertil Steril. 2017;107(3):664–70.
Druckenmiller S, Noyes N, Sutter ME, McCulloh DH, Grifo JA. Morphology still matters when selecting euploid embryos: inner cell mass (ICM) and trophectoderm (TE) are predictive of pregnancy outcomes. Fertil Steril. 2019;112(3, Supplement):e11–e2.
Khosravi P, Kazemi E, Zhan Q, Malmsten JE, Toschi M, Zisimopoulos P, et al. Deep learning enables robust assessment and selection of human blastocysts after in vitro fertilization. NPJ Digit Med. 2019;2:21.
Curchoe CL, Bormann CL. Artificial intelligence and machine learning for human reproduction and embryology presented at ASRM and ESHRE 2018. J Assist Reprod Genet. 2019;36(4):591–600.
Tran A, Cooke S, Illingworth PJ, Gardner DK. Artificial intelligence as a novel approach for embryo selection. Fertil Steril. 2018;110(4):e430.
Rocha C, Nogueira MG, Zaninovic N, Hickman C. Is AI assessment of morphokinetic data and digital image analysis from time-lapse culture predictive of implantation potential of human embryos? Fertil Steril. 2018;110(4):e373.
Sekhon L, Lee JA, Flisser E, Copperman AB, Stein D. Blastocyst vitrification, cryostorage and warming does not affect live birth rate, infant birth weight or timing of delivery. Reprod BioMed Online. 2018;37(1):33–42.
Hernandez-Nieto C, Lee JA, Slifkin R, Sandler B, Copperman AB, Flisser E. What is the reproductive potential of day 7 euploid embryos? Hum Reprod. 2019;34(9):1697–706.
Arce JC, Ziebe S, Lundin K, Janssens R, Helmgaard L, Sørensen P. Interobserver agreement and intraobserver reproducibility of embryo quality assessments. Hum Reprod. 2006;21(8):2141–8.
Dimitriadis I, Bormann CL, Thirumalaraju P, Kanakasabapathy M, Gupta R, Pooniwala R, et al. Artificial intelligence-enabled system for embryo classification and selection based on image analysis. Fertil Steril. 2019;111(4):e21.
VerMilyea M, Hall JMM, Diakiw SM, Johnston A, Nguyen T, Perugini D, et al. Development of an artificial intelligence-based assessment model for prediction of embryo viability using static images captured by optical light microscopy during IVF. Hum Reprod. 2020;35(4):770–84.
Tran D, Cooke S, Illingworth PJ, Gardner DK. Deep learning as a predictive tool for fetal heart pregnancy following time-lapse incubation and blastocyst transfer. Hum Reprod. 2019;34(6):1011–8.
Bori L, Meseguer F, Valera Cerda MA, Alegre L, Tejera A, Remohi J, Meseguer M. A universal algorithm is available in last generation time-lapse incubators: embryo score provided by the KIDScoreD5 is strongly correlated with chromosomal status and clinical outcomes. Presented at the 36th Annual Meeting of European Society of Human Reproduction and Embryology. 2020.
Acknowledgements
The authors thank the physicians, embryologists, research, and other staff members at the study site and affiliated hospitals for their valuable contributions to the development of this manuscript.
Code availability
Not applicable.
Author information
Authors and Affiliations
Contributions
J.F., C.H.N., A.C., and T.N. provided substantial contribution to the design of the study. J.F., C.H.N., D.G. R.M.R, R.S., and C.B.J. collected and analyzed the data. J.F., C.H.N., J.A.L., A.C., and C.B.J. drafted the manuscript. All authors interpreted the data, revised the work, and approved the final submitted version. All authors contributed to the study conception and design. Material preparation and data collection was performed by Jenna Friedenthal, Dmitry Gounko, Rose Marie Roth, and Richard Slifkin. Formal analysis was performed by Jenna Friedenthal, Carlos Hernandez-Nieto, and Dmitry Gounko. Original draft preparation was performed by Jenna Friedenthal; review and editing was performed by all authors. Christine Briton-Jones, Taraneh Nazem, Joseph A. Lee, and Alan Copperman made significant editorial contributions to the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Committee (IRB# 18-00452) of the Icahn School of Medicine at Mount Sinai approved this study.
Consent to participate
Not applicable. The Human Investigation Committee (IRB# 18-00452) of the Icahn School of Medicine at Mount Sinai approved this retrospective chart review study.
Consent to publish
Not applicable. The Human Investigation Committee (IRB# 18-00452) of the Icahn School of Medicine at Mount Sinai approved this retrospective chart review study.
Conflict of interest
Alan Copperman is a board member of Sema4 Genomics and Progyny and possesses stock/stock options in Sema4 Genomics and Progyny. The other coauthors declare no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Friedenthal, J., Hernandez-Nieto, C., Roth, R.M. et al. Clinical implementation of algorithm-based embryo selection is associated with improved pregnancy outcomes in single vitrified warmed euploid embryo transfers. J Assist Reprod Genet 38, 1647–1653 (2021). https://doi.org/10.1007/s10815-021-02203-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-021-02203-3