Abstract
Purpose
The objective of this virtual study was to simulate a full cycle and assess the costs and benefits to a couple with a reciprocal translocation, using current techniques for preimplantation genetic testing and comparing reporting every chromosome with only reporting those involved in the rearrangement.
Methods
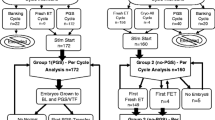
A simulation was constructed for women under the age of 35 years, where vitrified-warmed embryos were transferred one at a time in a first full cycle following preimplantation genetic testing using next-generation sequencing and sampling the trophectoderm at the blastocyst stage. The effect on pregnancy outcomes (live birth, clinical miscarriage and biochemical pregnancy) was evaluated for different reporting strategies for embryo transfer to (i) report only the rearranged chromosomes and transfer embryos with a normal or balanced test result for the translocation (targeted), or (ii) report every chromosome and exclude from transfer all embryos with an abnormal test result (exclusion), or (iii) exclude only those consistent with an unbalanced translocation and/or unrelated non-mosaic whole-chromosome aneuploidy and assign those with samples consistent with a normal or balanced translocation complement and unrelated mosaic aneuploidy or segmental imbalance (embryos of uncertain reproductive potential) a lower transfer priority (ranking). The number of individual women whom might benefit by avoiding an adverse pregnancy outcome (biochemical pregnancy, clinical miscarriage) or be disadvantaged by not achieving a live birth was evaluated. The financial cost of the different reporting strategies and the time taken to complete a cycle were also considered.
Results
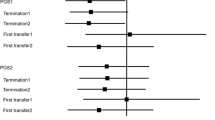
The simulation showed that compared to only reporting the translocation chromosomes (targeted reporting), testing every chromosome and ranking embryos by test result for transfer was a cost-effective strategy to avoid an adverse pregnancy without compromising the chance of a live birth. Excluding from transfer embryos with a test result consistent with a normal or balanced translocation complement of uncertain reproductive potential was an inferior strategy because it resulted in fewer live births from a full cycle. Reporting only the translocation chromosomes was an inferior strategy because it was less effective than ranked reporting of every chromosome to avoid an adverse pregnancy. Compared to targeted reporting, the ranked and exclusion strategies marginally reduced the overall cost and time taken to complete a full cycle. The ranking strategy avoided 1 adverse pregnancy for 12 cycles started, compared to 1 in 10 for the exclusion strategy which also resulted in 1 in 22 fewer women achieving a live birth. A minority (< 10%) of couples benefited by avoiding at least 1 adverse pregnancy whilst also reducing the time and cost for a complete cycle; most (> 70% ) couples received no benefit additional to targeted reporting and had the same outcome for pregnancy, time and cost.
Conclusions
The primary objective of PGT-SR for couples with a reciprocal translocation is to avoid a pregnancy with a chromosomally unbalanced product of the translocation and to reduce the risk of miscarriage, at least to that expected for couples with normal karyotypes. Trophectoderm sampling at the blastocyst stage with testing using NGS is an effective approach; however, ranking and excluding from transfer embryos with abnormal test results for unrelated chromosomes is problematical and is likely to be detrimental to achieving a live birth. Targeted reporting, where only the results of the chromosomes involved in the translocation are known, should be preferred to achieve a live birth. A best effort should be made to follow up and investigate all pregnancies following PGT-SR. Once the reproductive outcome is known (biochemical pregnancy, clinical pregnancy, live birth), the chromosomes unrelated to the rearrangement can be analysed as an experimental study. The risk/benefit of avoiding an adverse pregnancy vs reducing the chance of a healthy delivery should be a decision for each individual couple and informed by appropriate genetic counselling for their specific translocation and history.


Similar content being viewed by others
Data Availability
Supplementary file provided.
Code availability
Not applicable.
References
Munné S, Kaplan B, Frattarelli JL, Child T, Nakhuda G, Shamma FN, et al. STAR Study Group. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril. 2019;112:1071–9.
Tiegs AW, Tao X, Zhan Y, Whitehead C, Kim J, Hanson B, et al. A multicenter, prospective, blinded, nonselection study evaluating the predictive value of an aneuploid diagnosis using a targeted next-generation sequencing-based preimplantation genetic testing for aneuploidy assay and impact of biopsy. Fertil Steril. 2020;115:627–37. https://doi.org/10.1016/j.fertnstert.2020.07.052.
Gleicher N, Barad DH, Ben-Rafael Z, Glujovsky D, Mochizuki L, Modi D, et al. International Do No Harm Group in IVF (IDNHG-IVF). Commentary on two recently published formal guidelines on management of “mosaic” embryos after preimplantation genetic testing for aneuploidy (PGT-A). Reprod Biol Endocrinol. 2021;19:23. https://doi.org/10.1186/s12958-021-00716-1.
Yuan P, Zheng L, Ou S, Zhao H, Li R, Luo H, et al. Evaluation of chromosomal abnormalities from preimplantation genetic testing to the reproductive outcomes: a comparison between three different structural rearrangements based on next-generation sequencing. J Assist Reprod Genet. 2021;38:709–18. https://doi.org/10.1007/s10815-020-02053-5.
Cheng D, Hu L, Gong F, Yuan S, Luo K, Wu X, et al. Clinical outcomes following preimplantation genetic testing and microdissecting junction region in couples with balanced chromosome rearrangement. J Assist Reprod Genet. 2021;38:735–42. https://doi.org/10.1007/s10815-020-02052-6.
Scriven PN. Towards a better understanding of preimplantation genetic screening for aneuploidy: insights from a virtual trial for women under the age of 40 when transferring embryos one at a time. Reprod Biol Endocrinol. 2017;15:49.
Guy’s and St Thomas’ Assisted Conception Unit Private Healthcare. https://guysandstthomasprivatehealthcare.co.uk/wp-content/uploads/2018/11/ACU-Self-Funding-Price-List.pdf. Accessed 18 Feb 2021.
Uitenbroek DG: SISA - Two by two table. http://www.quantitativeskills.com/sisa/statistics/twoby2.htm . Accessed 18 Feb 2021.
Scriven PN, Flinter FA, Khalaf Y, Lashwood A, Mackie Ogilvie C. Benefits and drawbacks of preimplantation genetic diagnosis (PGD) for reciprocal translocations: lessons from a prospective cohort study. Eur J Hum Genet. 2013;21:1035–41.
Harper J, Jackson E, Sermon K, Aitken RJ, Harbottle S, Mocanu E, et al. Adjuncts in the IVF laboratory: where is the evidence for ‘add-on’ interventions? Hum Reprod. 2017;32:485–91.
Gleicher N, Patrizio P, Brivanlou A. preimplantation genetic testing for aneuploidy - a castle built on sand. Trends Mol Med. 2021 Jan 11:S1471-4914(20)30313-0. https://doi.org/10.1016/j.molmed.2020.11.009.
Midro AT, Stengel-Rutkowski S, Stene J. Experiences with risk estimates for carriers of chromosomal reciprocal translocations. Clin Genet. 1992;41:113–22.
Franssen MT, Korevaar JC, van der Veen F, Leschot NJ, Bossuyt PM, Goddijn M. Reproductive outcome after chromosome analysis in couples with two or more miscarriages: index [corrected]-control study. BMJ. 2006;332:759–63.
Author information
Authors and Affiliations
Contributions
The author is responsible for the content and writing of the paper.
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The author declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(XLSX 2216 kb)
Rights and permissions
About this article
Cite this article
Scriven, P.N. PGT-SR (reciprocal translocation) using trophectoderm sampling and next-generation sequencing: insights from a virtual trial. J Assist Reprod Genet 38, 1971–1978 (2021). https://doi.org/10.1007/s10815-021-02174-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-021-02174-5