Abstract
Purpose
To study the relationship between the migration speed of nucleolus precursor bodies (NPBs) in male and female pronuclei (mPN; fPN) and human embryo development during assisted reproduction.
Methods
The migration speed of 263 NPBs from 47 zygotes was quantitated, and embryonic development was observed until the blastocyst stage. The central coordinates of mPN, fPN, and NPBs were noted at multiple timepoints. Then, the distance traveled by the NPBs between two sequential images was measured, and migration speed was calculated. Additionally, we investigated the relationship between NPB migration speed and ploidy status (N = 33) or live birth/ongoing pregnancy (LB/OP) (N = 60) after assisted reproduction.
Results
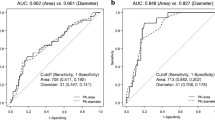
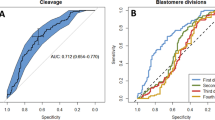
The NPB migration speed in both mPN and fPN was significantly faster in the zygotes that developed into blastocysts (N = 25) than that in the zygotes that arrested (N = 22). The timing of blastulation was negatively correlated with NPB migration speed in the mPN. Faster NPB migration was significantly correlated with LB/OP. In multivariate logistic analysis, NPB migration speed in the mPN was the only morphokinetic parameter associated with LB/OP. In a receiver-operating characteristic curve analysis of LB/OP by the NPB migration speed in the mPN, the cut-off value was 4.56 μm/h. When this cut-off value was applied to blastocysts with preimplantation genetic testing for aneuploidy, 100% of the blastocysts faster than or equal to the cut-off value were euploid.
Conclusion
The NPBs migrated faster in zygotes having the potential to develop into a blastocyst, and eventually into a baby. This predictor could be an attractive marker for non-invasive embryo selection.



Similar content being viewed by others
References
Berry J, Weber SC, Vaidya N, Haataja M, Brangwynne CP. RNA transcription modulates phase transition-driven nuclear body assembly. Proc Natl Acad Sci U S A. 2015;112:E5237–45.
Kyogoku H, Kitajima TS, Miyano T. Nucleolus precursor body (NPB): a distinct structure in mammalian oocytes and zygotes. Nucleus. 2014;5:493–8.
Fulka H, Rychtarova J, Loi P. The nucleolus-like and precursor bodies of mammalian oocytes and embryos and their possible role in post-fertilization centromere remodelling. Biochem Soc Trans. 2020;48:581–93.
Fulka H, Aoki F. Nucleolus precursor bodies and ribosome biogenesis in early mammalian embryos: old theories and new discoveries. Biol Reprod. 2016;94:143.
Kresoja-Rakic J, Santoro R. Nucleolus and rRNA gene chromatin in early embryo development. Trends Genet. 2019;35:868–79.
Ogushi S, Palmieri C, Fulka H, Saitou M, Miyano T, Fulka J Jr. The maternal nucleolus is essential for early embryonic development in mammals. Science. 2008;319:613–6.
Otsuki J, Iwasaki T, Tsuji Y, Katada Y, Sato H, Tsutsumi Y, et al. Potential of zygotes to produce live births can be identified by the size of the male and female pronuclei just before their membranes break down. Reprod Med Biol. 2017;16:200–5.
Otsuki J, Iwasaki T, Enatsu N, Katada Y, Furuhashi K, Shiotani M. Noninvasive embryo selection: kinetic analysis of female and male pronuclear development to predict embryo quality and potential to produce live birth. Fertil Steril. 2019;112:874–81.
Araki E, Itoi F, Honnma H, Asano Y, Oguri H, Nishikawa K. Correlation between the pronucleus size and the potential for human single pronucleus zygotes to develop into blastocysts: 1PN zygotes with large pronuclei can expect an embryo development to the blastocyst stage that is similar to the development of 2PN zygotes. J Assist Reprod Genet. 2018;35:817–23.
Coticchio G, Mignini Renzini M, Novara PV, Lain M, De Ponti E, Turchi D, et al. Focused time-lapse analysis reveals novel aspects of human fertilization and suggests new parameters of embryo viability. Hum Reprod. 2018;33:23–31.
ALPHA Scientists In Reproductive Medicine; ESHRE Special Interest Group Embryology. Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Reprod BioMed Online. 2011;22:632–46.
Mio Y. Morphological analysis of human embryonic development using time-lapse cinematography. J Mamm Ova Res. 2006;23:27–35.
Montag M, Liebenthron J, Köster M. Which morphological scoring system is relevant in human embryo development? Placenta. 2011;32(Suppl 3):S252–6.
Inoue T, Yamashita Y, Tsujimoto Y, Yamamoto S, Taguchi S, Hirao K, et al. The association of follicular fluid volume with human oolemma stretchability during intracytoplasmic sperm injection. Clin Exp Reprod Med. 2017;44:126–31.
Inoue T, Sugimoto H, Okubo K, Emi N, Matsushita Y, Kojima K, et al. Successful pregnancy after intracytoplasmic sperm injection with testicular spermatozoa transported only under refrigeration. Reprod Med Biol. 2010;9:173–7.
Gardner DK, Schoolcraft WB. In vitro culture of human blastocyst. In: Jansen R, Mortimer D, editors. Towards reproductive certainty: infertility and genetics beyond 1999. Carnforth: Parthenon Press; 1999. p. 377–88.
Kuwayama M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: the Cryotop method. Theriogenology. 2007;67:73–80.
World Health Organization. International statistical classification of diseases and related health problems. 10th revision, Fifth edition, 2016.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91.
Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale: Lawrence Erlbaum; 1988.
Cohen J. A power primer. Psychol Bull. 1992;112:155–9.
Ogushi S, Saitou M. The nucleolus in the mouse oocyte is required for the early step of both female and male pronucleus organization. J Reprod Dev. 2010;56:495–501.
Jachowicz JW, Santenard A, Bender A, Muller J, Torres-Padilla ME. Heterochromatin establishment at pericentromeres depends on nuclear position. Genes Dev. 2013;27:2427–32.
Fulka H, Langerova A. The maternal nucleolus plays a key role in centromere satellite maintenance during the oocyte to embryo transition. Development. 2014;141:1694–704.
Kyogoku H, Fulka J Jr, Wakayama T, Miyano T. De novo formation of nucleoli in developing mouse embryos originating from enucleolated zygotes. Development. 2014;141:2255–9.
Zatsepina O, Baly C, Chebrout M, Debey P. The step-wise assembly of a functional nucleolus in preimplantation mouse embryos involves the cajal (coiled) body. Dev Biol. 2003;253:66–83.
Lavrentyeva E, Shishova K, Kagarlitsky G, Zatsepina O. Localisation of RNAs and proteins in nucleolar precursor bodies of early mouse embryos. Reprod Fertil Dev. 2017;29:509–20.
Koné MC, Fleurot R, Chebrout M, Debey P, Beaujean N, Bonnet-Garnier A. Three-dimensional distribution of UBF and Nopp140 in relationship to ribosomal DNA transcription during mouse preimplantation development. Biol Reprod. 2016;94:95.
Bonnet-Garnier A, Kiêu K, Aguirre-Lavin T, Tar K, Flores P, Liu Z, et al. Three-dimensional analysis of nuclear heterochromatin distribution during early development in the rabbit. Chromosoma. 2018;127:387–403.
Svarcova O, Dinnyes A, Polgar Z, Bodo S, Adorjan M, Meng Q, et al. Nucleolar re-activation is delayed in mouse embryos cloned from two different cell lines. Mol Reprod Dev. 2009;76:132–41.
García-Rodríguez A, Gosálvez J, Agarwal A, Roy R, Johnston S. DNA damage and repair in human reproductive cells. Int J Mol Sci. 2018;20:31.
Hinz JM, Czaja W. Facilitation of base excision repair by chromatin remodeling. DNA Repair (Amst). 2015;36:91–7.
Kawamura K, Qi F, Meng Q, Hayashi I, Kobayashi J. Nucleolar protein nucleolin functions in replication stress-induced DNA damage responses. J Radiat Res. 2019;60:281–8.
Derijck A, van der Heijden G, Giele M, Philippens M, de Boer P. DNA double-strand break repair in parental chromatin of mouse zygotes, the first cell cycle as an origin of de novo mutation. Hum Mol Genet. 2008;17:1922–37.
Wdowiak A, Bakalczuk S, Bakalczuk G. The effect of sperm DNA fragmentation on the dynamics of the embryonic development in intracytoplasmatic sperm injection. Reprod Biol. 2015;15:94–100.
Horta F, Catt S, Ramachandran P, Vollenhoven B, Temple-Smith P. Female ageing affects the DNA repair capacity of oocytes in IVF using a controlled model of sperm DNA damage in mice. Hum Reprod. 2020;35:529–44.
Zhang L, Wei D, Zhu Y, Gao Y, Yan J, Chen ZJ. Rates of live birth after mosaic embryo transfer compared with euploid embryo transfer. J Assist Reprod Genet. 2019;36:165–72.
Boynukalin FK, Gultomruk M, Cavkaytar S, Turgut E, Findikli N, Serdarogullari M, et al. Parameters impacting the live birth rate per transfer after frozen single euploid blastocyst transfer. PLoS One. 2020;15:e0227619.
Pederson T. Growth factors in the nucleolus? J Cell Biol. 1998;143:279–81.
Scott L. Pronuclear scoring as a predictor of embryo development. Reprod BioMed Online. 2003;6:201–14.
Aguilar J, Motato Y, Escribá MJ, Ojeda M, Muñoz E, Meseguer M. The human first cell cycle: impact on implantation. Reprod BioMed Online. 2014;28:475–84.
Ezoe K, Ohata K, Morita H, Ueno S, Miki T, Okimura T, et al. Prolonged blastomere movement induced by the delay of pronuclear fading and first cell division adversely affects pregnancy outcomes after fresh embryo transfer on day 2: a time-lapse study. Reprod BioMed Online. 2019;38:659–68.
Leaver M, Wells D. Non-invasive preimplantation genetic testing (niPGT): the next revolution in reproductive genetics? Hum Reprod Update. 2020;26:16–42.
Vera-Rodriguez M, Diez-Juan A, Jimenez-Almazan J, Martinez S, Navarro R, Peinado V, et al. Origin and composition of cell-free DNA in spent medium from human embryo culture during preimplantation development. Hum Reprod. 2018;33:745–56.
Hammond ER, McGillivray BC, Wicker SM, Peek JC, Shelling AN, Stone P, et al. Characterizing nuclear and mitochondrial DNA in spent embryo culture media: genetic contamination identified. Fertil Steril. 2017;107:220–8.e5.
Papale L, Fiorentino A, Montag M, Tomasi G. The zygote. Hum Reprod. 2012;27(Suppl 1):i22–49.
Wei D, Liu JY, Sun Y, Shi Y, Zhang B, Liu JQ, et al. Frozen versus fresh single blastocyst transfer in ovulatory women: a multicentre, randomised controlled trial. Lancet. 2019;393:1310–8.
Santos-Ribeiro S, Mackens S, Popovic-Todorovic B, Racca A, Polyzos NP, Van Landuyt L, et al. The freeze-all strategy versus agonist triggering with low-dose hCG for luteal phase support in IVF/ICSI for high responders: a randomized controlled trial. Hum Reprod. 2020;35:2808–18.
Acknowledgements
We thank Kayoko Hirao for performing ICSI, cryopreservation, and warming. We thank Shogo Shiratsuki, PhD (Merck Biopharma Co., Ltd.) for critical review and scientific discussion on the manuscript. We thank Shannon Wyszomierski, PhD for editing the manuscript.
Author information
Authors and Affiliations
Contributions
T. I. designed this study, performed tracking NPBs and PNs, collected and analyzed data, and drafted the manuscript. S. T. annotated morphokinetic parameters. Y. T. performed biopsy for PGT-A. T. I., S. T., and Y. T. performed ICSI, cryopreservation, and warming. M. U. contributed to the data analysis and critically reviewed the manuscript. K. M. and Y. Y. performed ovarian stimulation, oocyte retrieval, blastocyst transfer, and luteal support. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures were performed in accordance with the 1964 Helsinki declaration. This study was approved by the Umeda Fertility Clinic Institutional Review Board (181215).
Consent to participate
Informed consent was obtained from all patients.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Inoue, T., Taguchi, S., Uemura, M. et al. Migration speed of nucleolus precursor bodies in human male pronuclei: a novel parameter for predicting live birth. J Assist Reprod Genet 38, 1725–1736 (2021). https://doi.org/10.1007/s10815-021-02172-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-021-02172-7