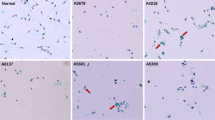
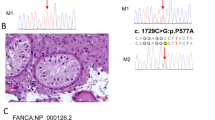
Abstract
Purpose
We aimed (1) to determine the molecular diagnosis rate and the recurrent causative genes of patients with non-obstructive azoospermia (NOA) using targeted next-generation sequencing (NGS) panel screening and (2) to discuss whether these genes help in the prognosis for microsurgical testicular sperm extraction (micro-TESE).
Methods
We used NGS panels to screen 668 Chinese men with NOA. Micro-TESE outcomes for six patients with pathogenic mutations were followed up. Functional assays were performed for two NR5A1 variants identified: p.I224V and p.R281C.
Results
Targeted NGS panel sequencing could explain 4/189 (2.1% by panel 1) or 10/479 (2.1% by panel 2) of the patients with NOA after exclusion of karyotype abnormalities and Y chromosome microdeletions. Almost all mutations detected were newly described except for NR5A1 p.R281C and TEX11 p.M156V. Two missense NR5A1 mutations—p.R281C and p.I244V—were proved to be deleterious by in vitro functional assays. Mutations in TEX11, TEX14, and NR5A1 genes are recurrent causes of NOA, but each gene explains only a very small percentage (less than 4/668; 0.6%). Only the patient with NR5A1 mutations produced viable spermatozoa through micro-TESE, but other patients with TEX11 and TEX14 had poor micro-TESE prognoses.
Conclusions
A targeted NGS panel is a feasible diagnostic method for patients with NOA. Because each gene implicated explains only a small proportion of such cases, more genes should be included to further increase the diagnostic rate. Considering previous reports, we suggest that only a few genes that are directly linked to meiosis can indicate poor micro-TESE prognosis, such as TEX11, TEX14, and SYCE1.


Similar content being viewed by others
References
Lotti F, Maggi M. Sexual dysfunction and male infertility. Nat Rev Urol. 2018;15:287–307.
WHO. WHO laboratory manual for the examination and processing of human semen. Fifth ed. Geneva: WHO; 2010.
Jarow JP, Espeland MA, Lipshultz LI. Evaluation of the azoospermic patient. J Urol. 1989;142:62–5.
Salonia A, Bettochi C, Carvalho J, Corona G, Jones TH, Kadioğlu A, et al. EAU guidelines on sexual and reproductive health 2020. In: European Association of Urology Guidelines. 2020 Edition., vol. presented at the EAU Annual Congress Amsterdam 2020. Arnhem, The Netherlands: European Association of Urology Guidelines Office; 2020.
Krausz C, Hoefsloot L, Simoni M, Tuttelmann F. European Academy of A, European Molecular Genetics Quality N. EAA/EMQN best practice guidelines for molecular diagnosis of Y-chromosomal microdeletions: state-of-the-art 2013. Andrology. 2014;2:5–19.
Van Assche E, Bonduelle M, Tournaye H, Joris H, Verheyen G, Devroey P, et al. Cytogenetics of infertile men. Hum Reprod. 1996;11(Suppl 4):1–24 discussion 25-6.
Liu XY, Zhang HY, Pang DX, Xue LT, Yang X, Li YS, et al. AZFa microdeletions: occurrence in Chinese infertile men and novel deletions revealed by semiconductor sequencing. Urology. 2017;107:76–81.
Kasak L, Laan M. Monogenic causes of non-obstructive azoospermia: challenges, established knowledge, limitations and perspectives. Hum Genet. 2021;140(1):135–154.
Matzuk MM, Lamb DJ. The biology of infertility: research advances and clinical challenges. Nat Med. 2008;14:1197–213.
Luo M, Yang F, Leu NA, Landaiche J, Handel MA, Benavente R, et al. MEIOB exhibits single-stranded DNA-binding and exonuclease activities and is essential for meiotic recombination. Nat Commun. 2013;4:2788.
Greenbaum MP, Yan W, Wu M-H, Lin Y-N, Agno JE, Sharma M, et al. TEX14 is essential for intercellular bridges and fertility in male mice. Proc Natl Acad Sci U S A. 2006;103:4982–7.
Maor-Sagie E, Cinnamon Y, Yaacov B, Shaag A, Goldsmidt H, Zenvirt S, et al. Deleterious mutation in SYCE1 is associated with non-obstructive azoospermia. J Assist Reprod Genet. 2015;32:887–91.
Miyamoto T, Hasuike S, Yogev L, Maduro MR, Ishikawa M, Westphal H, et al. Azoospermia in patients heterozygous for a mutation in SYCP3. Lancet. 2003;362:1714–9.
Yatsenko AN, Georgiadis AP, Ropke A, Berman AJ, Jaffe T, Olszewska M, et al. X-linked TEX11 mutations, meiotic arrest, and azoospermia in infertile men. N Engl J Med. 2015;372:2097–107.
Abou Alchamat G, Madania A, Alhalabi M. Mild androgen insensitivity syndrome (MAIS): the identification of c.1783C>T mutation in two unrelated infertile men. BMJ Case Rep. 2017;2017:bcr2017220361.
Fabbri-Scallet H, de Sousa LM, Maciel-Guerra AT, Guerra-Júnior G, de Mello MP. Mutation update for the NR5A1 gene involved in DSD and infertility. Hum Mutat. 2020;41:58–68.
Yatsenko AN, Roy A, Chen R, Ma L, Murthy LJ, Yan W, et al. Non-invasive genetic diagnosis of male infertility using spermatozoal RNA: KLHL10 mutations in oligozoospermic patients impair homodimerization. Hum Mol Genet. 2006;15:3411–9.
Kasak L, Punab M, Nagirnaja L, Grigorova M, Minajeva A, Lopes AM, et al. Bi-allelic recessive loss-of-function variants in FANCM cause non-obstructive azoospermia. Am J Hum Genet. 2018;103:200–12.
Choi Y, Jeon S, Choi M, Lee MH, Park M, Lee DR, et al. Mutations in SOHLH1 gene associate with nonobstructive azoospermia. Hum Mutat. 2010;31:788–93.
Pezzi N, Prieto I, Kremer L, Perez Jurado LA, Valero C, Del Mazo J, et al. STAG3, a novel gene encoding a protein involved in meiotic chromosome pairing and location of STAG3-related genes flanking the Williams-Beuren syndrome deletion. FASEB J. 2000;14:581–92.
Okutman O, Muller J, Baert Y, Serdarogullari M, Gultomruk M, Piton A, et al. Exome sequencing reveals a nonsense mutation in TEX15 causing spermatogenic failure in a Turkish family. Hum Mol Genet. 2015;24:5581–8.
Arafat M, Har-Vardi I, Harlev A, Levitas E, Zeadna A, Abofoul-Azab M, et al. Mutation in TDRD9 causes non-obstructive azoospermia in infertile men. J Med Genet. 2017;54:633–9.
Ayhan O, Balkan M, Guven A, Hazan R, Atar M, Tok A, et al. Truncating mutations in TAF4B and ZMYND15 causing recessive azoospermia. J Med Genet. 2014;51:239–44.
Cannarella R, Condorelli RA, Duca Y, La Vignera S, Calogero AE. New insights into the genetics of spermatogenic failure: a review of the literature. Hum Genet. 2019;138:125–40.
Robay A, Abbasi S, Akil A, El-Bardisi H, Arafa M, Crystal RG, et al. A systematic review on the genetics of male infertility in the era of next-generation sequencing. Arab J Urol. 2018;16:53–64.
Oud MS, Volozonoka L, Smits RM, Vissers L, Ramos L, Veltman JA. A systematic review and standardized clinical validity assessment of male infertility genes. Hum Reprod. 2019;34:932–41.
Smith ED, Radtke K, Rossi M, Shinde DN, Darabi S, El-Khechen D, et al. Classification of genes: standardized clinical validity assessment of gene-disease associations aids diagnostic exome analysis and reclassifications. Hum Mutat. 2017;38:600–8.
Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–60.
Lai Z, Markovets A, Ahdesmaki M, Chapman B, Hofmann O, McEwen R, et al. VarDict: a novel and versatile variant caller for next-generation sequencing in cancer research. Nucleic Acids Res. 2016;44:e108.
Li Q, Wang K. InterVar: clinical interpretation of genetic variants by the 2015 ACMG-AMP guidelines. Am J Hum Genet. 2017;100:267–80.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24.
Venkatraman ES, Olshen AB. A faster circular binary segmentation algorithm for the analysis of array CGH data. Bioinformatics. 2007;23:657–63.
Thorvaldsdottir H, Robinson JT, Mesirov JP. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform. 2013;14:178–92.
Riggs ER, Andersen EF, Cherry AM, Kantarci S, Kearney H, Patel A, et al. Technical standards for the interpretation and reporting of constitutional copy-number variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics (ACMG) and the Clinical Genome Resource (ClinGen). Genet Med. 2020;22:245–57.
Bashamboo A, Ferraz-de-Souza B, Lourenco D, Lin L, Sebire NJ, Montjean D, et al. Human male infertility associated with mutations in NR5A1 encoding steroidogenic factor 1. Am J Hum Genet. 2010;87:505–12.
Lin L, Achermann JC. Steroidogenic factor-1 (SF-1, Ad4BP, NR5A1) and disorders of testis development. Sex Dev. 2008;2:200–9.
Chen S, Wang G, Zheng X, Ge S, Dai Y, Ping P, et al. Whole-exome sequencing of a large chinese azoospermia and severe oligospermia cohort identifies novel infertility causative variants and genes. Hum Mol Genet. 2020;29(14):2451–2459.
Rocca MS, Msaki A, Ghezzi M, Cosci I, Pilichou K, Celeghin R, et al. Development of a novel next-generation sequencing panel for diagnosis of quantitative spermatogenic impairment. J Assist Reprod Genet. 2020;37:753–62.
Fakhro KA, Elbardisi H, Arafa M, Robay A, Rodriguez-Flores JL, Al-Shakaki A, et al. Point-of-care whole-exome sequencing of idiopathic male infertility. Genet Med. 2018;20:1365–73.
Araujo TF, Friedrich C, Grangeiro CHP, Martelli LR, Grzesiuk JD, Emich J, et al. Sequence analysis of 37 candidate genes for male infertility: challenges in variant assessment and validating genes. Andrology. 2020;8:434–41.
Buonocore F, Clifford-Mobley O, King TFJ, Striglioni N, Man E, Suntharalingham JP, et al. Next-generation sequencing reveals novel genetic variants (SRY, DMRT1, NR5A1, DHH, DHX37) in adults with 46,XY DSD. J Endocr Soc. 2019;3:2341–60.
Zhe J, Ye D, Chen X, Liu Y, Zhou X, Li Y, et al. Consanguineous Chinese familial study reveals that a gross deletion that includes the SYCE1 gene region is associated with premature ovarian insufficiency. Reprod Sci. 2020;27:461–7.
Yang F, Gell K, van der Heijden GW, Eckardt S, Leu NA, Page DC, et al. Meiotic failure in male mice lacking an X-linked factor. Genes Dev. 2008;22:682–91.
Dunne OM, Davies OR. Molecular structure of human synaptonemal complex protein SYCE1. Chromosoma. 2019;128:223–36.
Gershoni M, Hauser R, Yogev L, Lehavi O, Azem F, Yavetz H, et al. A familial study of azoospermic men identifies three novel causative mutations in three new human azoospermia genes. Genet Med. 2017;19:998–1006.
Wang Q, Ghadessy FJ, Trounson A, de Kretser D, McLachlan R, Ng SC, et al. Azoospermia associated with a mutation in the ligand-binding domain of an androgen receptor displaying normal ligand binding, but defective trans-activation. J Clin Endocrinol Metab. 1998;83:4303–9.
Chang CS, Kokontis J, Liao ST. Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science. 1988;240:324–6.
Tremblay JJ, Viger RS. A mutated form of steroidogenic factor 1 (SF-1 G35E) that causes sex reversal in humans fails to synergize with transcription factor GATA-4. J Biol Chem. 2003;278:42637–42.
Hiort O, Klauber G, Cendron M, Sinnecker GH, Keim L, Schwinger E, et al. Molecular characterization of the androgen receptor gene in boys with hypospadias. Eur J Pediatr. 1994;153:317–21.
Zare-Abdollahi D, Safari S, Mirfakhraie R, Movafagh A, Bastami M, Azimzadeh P, et al. Mutational screening of the NR5A1 in azoospermia. Andrologia. 2015;47:395–401.
Hasani N, Mohseni Meybodi A, Rafaee A, Sadighi Gilani MA, Mohammadzadeh R, Sabbaghian M. Spermatogenesis disorder is associated with mutations in the ligand-binding domain of an androgen receptor. Andrologia. 2019;51:e13376.
Massin N, Bry H, Vija L, Maione L, Constancis E, Haddad B, et al. Healthy birth after testicular extraction of sperm and ICSI from an azoospermic man with mild androgen insensitivity syndrome caused by an androgen receptor partial loss-of-function mutation. Clin Endocrinol. 2012;77:593–8.
Massin N, Bry-Gauillard H, Abirached F, Bassam H, Claessens F, Young J. Androgen receptor gene mutation and partial androgen insensitivity syndrome: birth after testicular sperm extraction and ICSI. Fertil Steril. 2007;88:S387.
ElSheikh MG, Hosny MB, Elshenoufy A, Elghamrawi H, Fayad A, Abdelrahman S. Combination of vitamin E and clomiphene citrate in treating patients with idiopathic oligoasthenozoospermia: a prospective, randomized trial. Andrology. 2015;3:864–7.
Yan W, Si Y, Slaymaker S, Li J, Zheng H, Young DL, et al. Zmynd15 encodes a histone deacetylase-dependent transcriptional repressor essential for spermiogenesis and male fertility. J Biol Chem. 2010;285:31418–26.
Yan W, Ma L, Burns KH, Matzuk MM. Haploinsufficiency of kelch-like protein homolog 10 causes infertility in male mice. Proc Natl Acad Sci U S A. 2004;101:7793–8.
Johnsen SG. Testicular biopsy score count--a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones. 1970;1:2–25.
Acknowledgments
The authors thank all enrolled patients. The authors also thank Li Zhang and Changquan Guo from Nuprobe company for analyzing data.
Funding
This study was supported by grants from the National Natural Science Foundation of China (81971376), a grant from the science and technology project of Pudong New Area Health and Family Planning Commission, Shanghai, China (XG8300000-2017-364), a grant from the Health Commission of Pudong New Area, Shanghai, P. R. China (PW2020D-7), a grant from Shanghai Municipal Health Commission for advanced and suitable technology promotion projects (2019SY056), and Clinical Research Plan of SHDC (No. SHDC2020CR4035).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(XLSX 9 kb).
Rights and permissions
About this article
Cite this article
An, M., Liu, Y., Zhang, M. et al. Targeted next-generation sequencing panel screening of 668 Chinese patients with non-obstructive azoospermia. J Assist Reprod Genet 38, 1997–2005 (2021). https://doi.org/10.1007/s10815-021-02154-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-021-02154-9