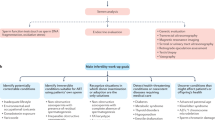
Abstract
Semen analysis is the cornerstone of evaluating male infertility, but it is imperfect and insufficient to diagnose male infertility. As a result, about 20% of infertile males have undetermined infertility, a term encompassing male infertility with an unknown underlying cause. Undetermined male infertility includes two categories: (i) idiopathic male infertility—infertile males with abnormal semen analyses with an unknown cause for that abnormality and (ii) unexplained male infertility—males with “normal” semen analyses who are unable to impregnate due to unknown causes. The treatment of males with undetermined infertility is limited due to a lack of understanding the frequency of general sperm defects (e.g., number, motility, shape, viability). Furthermore, there is a lack of trusted, quantitative, and predictive diagnostic tests that look inside the sperm to quantify defects such as DNA damage, RNA abnormalities, centriole dysfunction, or reactive oxygen species to discover the underlying cause. To better treat undetermined male infertility, further research is needed on the frequency of sperm defects and reliable diagnostic tools that assess intracellular sperm components must be developed. The purpose of this review is to uniquely create a paradigm of thought regarding categories of male infertility based on intracellular and extracellular features of semen and sperm, explore the prevalence of the various categories of male factor infertility, call attention to the lack of standardization and universal application of advanced sperm testing techniques beyond semen analysis, and clarify the limitations of standard semen analysis. We also call attention to the variability in definitions and consider the benefits towards undetermined male infertility if these gaps in research are filled.
Graphical abstract



Similar content being viewed by others
References
Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med. 2012;9(12):e1001356.
Thoma ME, et al. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013;99(5):1324–1331.e1.
Chandra A, Copen CE, Stephen EH. Infertility service use in the United States: Data from the National Survey of Family Growth, 1982-2010. Natl Health Stat Rep. 2014(73):1–21.
The epidemiology of infertility. Report of a WHO scientific group. World Health Organ Tech Rep Ser. 1975;582:1–37.
Philippov OS, Radionchenko AA, Bolotova VP, Voronovskaya NI, Potemkina TV. Estimation of the prevalence and causes of infertility in western Siberia. Bull World Health Organ. 1998;76(2):183–7.
Bayasgalan G, Naranbat D, Tsedmaa B, Tsogmaa B, Sukhee D, Amarjargal O, et al. Clinical patterns and major causes of infertility in Mongolia. J Obstet Gynaecol Res. 2004;30(5):386–93.
Ekwere P, et al. Infertility among Nigerian couples as seen in Calabar. Port Harcourt Med J. 2007;2.
Farhi J, Ben-Haroush A. Distribution of causes of infertility in patients attending primary fertility clinics in Israel. Isr Med Assoc J. 2011;13(1):51–4.
Oztekin U, et al. Evaluation of male infertility prevalence with clinical outcomes in Middle Anatolian region. Cureus. 2019;11(7):e5122.
Razzak AH, Wais SA. The infertile couple: a cohort study in Duhok, Iraq. East Mediterr Health J. 2002;8(2–3):234–8.
Thonneau P, Marchand S, Tallec A, Ferial ML, Ducot B, Lansac J, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988-1989). Hum Reprod. 1991;6(6):811–6.
Dyer SJ, Abrahams N, Hoffman M, van der Spuy ZM. ‘Men leave me as I cannot have children’: women’s experiences with involuntary childlessness. Hum Reprod. 2002;17(6):1663–8.
Turner KA, et al. Male infertility is a women’s health issue-research and clinical evaluation of male infertility is needed. Cells. 2020;9(4).
Smith S, Pfeifer SM, Collins JA. Diagnosis and management of female infertility. JAMA. 2003;290(13):1767–70.
Collins JA. Evidence-based infertility: evaluation of the female partner. Int Congr Ser. 2004;1266:57–62.
Sigman M, Lipshultz LI, Howards SS. In: Niederberger CS, Lipshultz LI, Howards SS, editors. Office evaluation of the subfertile male, in Infertility in the male. Cambridge: Cambridge University Press; 2009. p. 153–76.
Barratt CL. Semen analysis is the cornerstone of investigation for male infertility. Practitioner. 2007;251(1690):8–10, 12, 15-7.
Macomber D, Sanders MB. The spermatozoa count. N Engl J Med. 1929;200(19):981–4.
Patel AS, Leong JY, Ramasamy R. Prediction of male infertility by the World Health Organization laboratory manual for assessment of semen analysis: a systematic review. Arab J Urol. 2018;16(1):96–102.
Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HWG, Behre HM, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16(3):231–45.
Goyal R, Kotru M, Gogia A, Sharma S. Qualitative defects with normal sperm counts in a patient attending infertility clinic. Indian J Pathol Microbiol. 2018;61(2):233–5.
Murray KS, James A, McGeady JB, Reed ML, Kuang WW, Nangia AK. The effect of the new 2010 World Health Organization criteria for semen analyses on male infertility. Fertil Steril. 2012;98(6):1428–31.
Barbăroșie C, Agarwal A, Henkel R. Diagnostic value of advanced semen analysis in evaluation of male infertility. Andrologia. n/a(n/a):e13625.
Barroso G, Mercan R, Ozgur K, Morshedi M, Kolm P, Coetzee K, et al. Intra- and inter-laboratory variability in the assessment of sperm morphology by strict criteria: impact of semen preparation, staining techniques and manual versus computerized analysis. Hum Reprod. 1999;14(8):2036–40.
Filimberti E, Degl'Innocenti S, Borsotti M, Quercioli M, Piomboni P, Natali I, et al. High variability in results of semen analysis in andrology laboratories in Tuscany (Italy): the experience of an external quality control (EQC) programme. Andrology. 2013;1(3):401–7.
Jørgensen N, et al. Semen analysis performed by different laboratory teams: an intervariation study. Int J Androl. 1997;20(4):201–8.
Moazzam A, et al. From basic to contemporary semen analysis: limitations and variability. J Anim Plant Sci. 2015;25:328–36.
Auger J, Eustache F, Ducot B, Blandin T, Daudin M, Diaz I, et al. Intra- and inter-individual variability in human sperm concentration, motility and vitality assessment during a workshop involving ten laboratories. Hum Reprod. 2000;15(11):2360–8.
Brandriff B, Gordon L, Ashworth L, Watchmaker G, Moore D II, Wyrobek AJ, et al. Chromosomes of human sperm: variability among normal individuals. Hum Genet. 1985;70(1):18–24.
Brazil C. Practical semen analysis: from A to Z. Asian J Androl. 2010;12(1):14–20.
Guzick DS, Overstreet JW, Factor-Litvak P, Brazil CK, Nakajima ST, Coutifaris C, et al. Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med. 2001;345(19):1388–93.
Smith KD, Rodriguez-Rigau LJ, Steinberger E. Relation between indices of semen analysis and pregnancy rate in infertile couples*†. Fertil Steril. 1977;28(12):1314–9.
Group CCW. The current status and future of andrology: a consensus report from the Cairo workshop group. Andrology. 2020;8(1):27–52.
Thierry B. Integrating proximate and ultimate causation: just one more go! Curr Sci. 2005;89(7):1180–3.
Ring JD, Lwin AA, Köhler TS. Current medical management of endocrine-related male infertility. Asian J Androl. 2016;18(3):357–63.
Dubin L, Amelar RD. Etiologic factors in 1294 consecutive cases of male infertility *. Fertil Steril. 1971;22(8):469–74.
Esteves SC, Miyaoka R, Agarwal A. An update on the clinical assessment of the infertile male. [corrected]. Clinics (Sao Paulo). 2011;66(4):691–700.
Osegbe DN, Amaku EO. The causes of male infertility in 504 consecutive Nigerian patients. Int Urol Nephrol. 1985;17(4):349–58.
Al-Ali BM, et al. Clinical parameters and semen analysis in 716 Austrian patients with varicocele. Urology. 2010;75(5):1069–73.
Al-Ali BM, et al. Clinical and laboratory profiles of a large cohort of patients with different grades of varicocele. Central Eur J Urol. 2013;66(1):71–4.
Panner Selvam MK, et al. Etiologies of sperm DNA damage and its impact on male infertility. Andrologia. 2020;n/a(n/a):e13706.
Avidor-Reiss T, Carr A, Fishman EL. The sperm centrioles. Mol Cell Endocrinol. 2020;518:110987.
Grimes DA, Lopez LM. “Oligozoospermia,” “azoospermia,” and other semen-analysis terminology: the need for better science. Fertil Steril. 2007;88(6):1491–4.
Aduloju P, et al. Pattern of semen parameters and factors associated with infertility in male partners of infertile couples in Nigeria. Androl-Open Access. 2016;5:1.
Biradar KD. Male factor in infertility: study from a tertiary care hospital. Int J Reprod Contracept Obstet Gynecol. 2016;5(6). https://doi.org/10.18203/2320-1770.ijrcog20161710.
Butt F, Akram N. Semen analysis parameters: experiences and insight into male infertility at a tertiary care hospital in Punjab. J Pak Med Assoc. 2013;63(5):558–62.
Bartels I, Schlosser M, Bartz UG, Pauer HU. Paternal origin of trisomy 21 following intracytoplasmic sperm injection (ICSI). Hum Reprod. 1998;13(12):3345–6.
Plachot M, Belaisch-Allart J, Mayenga JM, Chouraqui A, Tesquier L, Serkine AM. Outcome of conventional IVF and ICSI on sibling oocytes in mild male factor infertility. Hum Reprod. 2002;17(2):362–9.
Rubio C, Gil-Salom M, Simón C, Vidal F, Rodrigo L, Mínguez Y, et al. Incidence of sperm chromosomal abnormalities in a risk population: relationship with sperm quality and ICSI outcome. Hum Reprod. 2001;16(10):2084–92.
Avidor-Reiss T, Mazur M, Fishman EL, Sindhwani P. The role of sperm centrioles in human reproduction - the known and the unknown. Front Cell Dev Biol. 2019;7:188.
Cassuto NG, Hazout A, Hammoud I, Balet R, Bouret D, Barak Y, et al. Correlation between DNA defect and sperm-head morphology. Reprod BioMed Online. 2012;24(2):211–8.
Kao S-H, Chao HT, Liu HW, Liao TL, Wei YH. Sperm mitochondrial DNA depletion in men with asthenospermia. Fertil Steril. 2004;82(1):66–73.
Sathananthan AH. Paternal centrosomal dynamics in early human development and infertility. J Assist Reprod Genet. 1998;15(3):129–39.
Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril. 2015;103(3):e18–25.
Huggins C, Scott WW, Heinen JH. Chemical composition of human semen and of the secretions of the prostate and seminal vesicles. Am J Physiol-Legacy Content. 1942;136(3):467–73.
Bracke A, Peeters K, Punjabi U, Hoogewijs D, Dewilde S. A search for molecular mechanisms underlying male idiopathic infertility. Reprod BioMed Online. 2018;36(3):327–39.
Calogero AE, Burrello N, de Palma A, Barone N, D'Agata R, Vicari E. Sperm aneuploidy in infertile men. Reprod BioMed Online. 2003;6(3):310–7.
Agarwal A and Said TM. Interpretation of basic semen analysis and advanced semen testing. 2011.
Lewis SEM. Is sperm evaluation useful in predicting human fertility? Reproduction. 2007;134(1):31–40.
Lewis SEM, Agbaje I, Alvarez J. Sperm DNA tests as useful adjuncts to semen analysis. Syst Biol Reprod Med. 2008;54(3):111–25.
Mau UA, Backert IT, Kaiser P, Kiesel L. Chromosomal findings in 150 couples referred for genetic counselling prior to intracytoplasmic sperm injection. Hum Reprod. 1997;12(5):930–7.
Meschede D, Lemcke B, Exeler JR, de Geyter C, Behre HM, Nieschlag E, et al. Chromosome abnormalities in 447 couples undergoing intracytoplasmic sperm injection--prevalence, types, sex distribution and reproductive relevance. Hum Reprod. 1998;13(3):576–82.
Testart J, Gautier E, Brami C, Rolet F, Sedbon E, Thebault A. Genetics: Intracytoplasmic sperm injection in infertile patients with structural chromosome abnormalities. Hum Reprod. 1996;11(12):2609–12.
Carrell DT, Wilcox AL, Lowy L, Peterson CM, Jones KP, Erickson L, et al. Elevated sperm chromosome aneuploidy and apoptosis in patients with unexplained recurrent pregnancy loss. Obstet Gynecol. 2003;101(6):1229–35.
Esquerré-Lamare C, Walschaerts M, Chansel Debordeaux L, Moreau J, Bretelle F, Isus F, et al. Sperm aneuploidy and DNA fragmentation in unexplained recurrent pregnancy loss: a multicenter case-control study. Basic Clin Androl. 2018;28(1):4.
Kohn TP, Kohn JR, Darilek S, Ramasamy R, Lipshultz L. Genetic counseling for men with recurrent pregnancy loss or recurrent implantation failure due to abnormal sperm chromosomal aneuploidy. J Assist Reprod Genet. 2016;33(5):571–6.
Practice Committee of the American Society for Reproductive Medicine. The clinical utility of sperm DNA integrity testing: a guideline. Fertil Steril. 2013;99(3):673–7.
Mehta A. Pros and cons of sperm DNA fragmentation testing: weighing the evidence. Transl Androl Urol. 2017;6(Suppl 4):S453–4.
Bungum M, Humaidan P, Axmon A, Spano M, Bungum L, Erenpreiss J, et al. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum Reprod. 2007;22(1):174–9.
Erenpreiss J, Spano M, Erenpreisa J, Bungum M, Giwercman A. Sperm chromatin structure and male fertility: biological and clinical aspects. Asian J Androl. 2006;8(1):11–29.
Giwercman A, Lindstedt L, Larsson M, Bungum M, Spano M, Levine RJ, et al. Sperm chromatin structure assay as an independent predictor of fertility in vivo: a case-control study. Int J Androl. 2010;33(1):e221–7.
Giwercman A, Richthoff J, Hjøllund H, Bonde JP, Jepson K, Frohm B, et al. Correlation between sperm motility and sperm chromatin structure assay parameters. Fertil Steril. 2003;80(6):1404–12.
Bungum M, Bungum L, Giwercman A. Sperm chromatin structure assay (SCSA): a tool in diagnosis and treatment of infertility. Asian J Androl. 2011;13(1):69–75.
Evenson DP. The Sperm Chromatin Structure Assay (SCSA®) and other sperm DNA fragmentation tests for evaluation of sperm nuclear DNA integrity as related to fertility. Anim Reprod Sci. 2016;169:56–75.
Evenson DP, et al. Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Hum Reprod. 1999;14(4):1039–49.
The clinical utility of sperm DNA integrity testing: a guideline. Fertil Steril. 2013;99(3):673–7.
Høst E, Lindenberg S, Smidt-Jensen S. DNA strand breaks in human spermatozoa: correlation with fertilization in vitro in oligozoospermic men and in men with unexplained infertility. Acta Obstet Gynecol Scand. 2000;79(3):189–93.
Oleszczuk K, Augustinsson L, Bayat N, Giwercman A, Bungum M. Prevalence of high DNA fragmentation index in male partners of unexplained infertile couples. Andrology. 2013;1(3):357–60.
Simon L, Proutski I, Stevenson M, Jennings D, McManus J, Lutton D, et al. Sperm DNA damage has a negative association with live-birth rates after IVF. Reprod BioMed Online. 2013;26(1):68–78.
Tan J, Taskin O, Albert A, Bedaiwy MA. Association between sperm DNA fragmentation and idiopathic recurrent pregnancy loss: a systematic review and meta-analysis. Reprod BioMed Online. 2019;38(6):951–60.
Avendaño C, Franchi A, Duran H, Oehninger S. DNA fragmentation of normal spermatozoa negatively impacts embryo quality and intracytoplasmic sperm injection outcome. Fertil Steril. 2010;94(2):549–57.
Tournaye H. Male factor infertility and ART. Asian J Androl. 2012;14(1):103–8.
Agarwal A, Cho CL, Esteves SC, Majzoub A. The price and value of sperm DNA fragmentation tests. Transl Androl Urol. 2017;6(Suppl 4):S597–9.
Chambers GM, Adamson GD, Eijkemans MJ. Acceptable cost for the patient and society. Fertil Steril. 2013;100(2):319–27.
Hammoud SS, Nix DA, Hammoud AO, Gibson M, Cairns BR, Carrell DT. Genome-wide analysis identifies changes in histone retention and epigenetic modifications at developmental and imprinted gene loci in the sperm of infertile men. Hum Reprod. 2011;26(9):2558–69.
Okada Y, Tateishi K, Zhang Y. Histone demethylase JHDM2A is involved in male infertility and obesity. J Androl. 2010;31(1):75–8.
Rajender S, Avery K, Agarwal A. Epigenetics, spermatogenesis and male infertility. Mutat Res/Rev Mutat Res. 2011;727(3):62–71.
Schon SB, Luense LJ, Wang X, Bartolomei MS, Coutifaris C, Garcia BA, et al. Histone modification signatures in human sperm distinguish clinical abnormalities. J Assist Reprod Genet. 2019;36(2):267–75.
Aoki VW, Emery BR, Liu L, Carrell DT. Protamine levels vary between individual sperm cells of infertile human males and correlate with viability and DNA integrity. J Androl. 2006;27(6):890–8.
Aoki VW, Liu L, Carrell DT. Identification and evaluation of a novel sperm protamine abnormality in a population of infertile males. Hum Reprod. 2005;20(5):1298–306.
Aoki VW, Moskovtsev SI, Willis J, Liu L, Mullen JB, Carrell DT. DNA integrity is compromised in protamine-deficient human sperm. J Androl. 2005;26(6):741–8.
Carrell DT, Emery BR, Hammoud S. The aetiology of sperm protamine abnormalities and their potential impact on the sperm epigenome. Int J Androl. 2008;31(6):537–45.
Li C, Zhou X. Gene transcripts in spermatozoa: markers of male infertility. Clin Chim Acta. 2012;413(13):1035–8.
Hamatani T. Human spermatozoal RNAs. Fertil Steril. 2012;97(2):275–81.
Jodar M, Kalko S, Castillo J, Ballescà JL, Oliva R. Differential RNAs in the sperm cells of asthenozoospermic patients. Hum Reprod. 2012;27(5):1431–8.
Jodar M, et al. Absence of sperm RNA elements correlates with idiopathic male infertility. Sci Transl Med. 2015;7(295):295re6.
Salas-Huetos A, Blanco J, Vidal F, Grossmann M, Pons MC, Garrido N, et al. Spermatozoa from normozoospermic fertile and infertile individuals convey a distinct miRNA cargo. Andrology. 2016;4(6):1028–36.
Jodar M, et al. Absence of sperm RNA elements correlates with idiopathic male infertility. Sci Transl Med. 2015;7(295):295re6.
Grow DR, Oehninger S, Seltman HJ, Toner JP, Swanson RJ, Kruger TF, et al. Sperm morphology as diagnosed by strict criteria: probing the impact of teratozoospermia on fertilization rate and pregnancy outcome in a large in vitro fertilization population. Fertil Steril. 1994;62(3):559–67.
Ombelet W, Fourie FR, Vandeput H, Bosmans E, Cox A, Janssen M, et al. Teratozoospermia and in-vitro fertilization: a randomized prospective study. Hum Reprod. 1994;9(8):1479–84.
Spiessens C, Vanderschueren D, Meuleman C, D'Hooghe T. Isolated teratozoospermia and intrauterine insemination. Fertil Steril. 2003;80(5):1185–9.
Wang G-H, et al. Impact of sperm malformation rate on ICSI clinical outcomes. Reprod Contracept. 2011;4:241–5.
Nie H, Tang Y, Qin W. Beyond acephalic spermatozoa: the complexity of intracytoplasmic sperm injection outcomes. Biomed Res Int. 2020;2020:6279795.
Fishman EL, Jo K, Nguyen QPH, Kong D, Royfman R, Cekic AR, et al. A novel atypical sperm centriole is functional during human fertilization. Nat Commun. 2018;9(1):2210.
Avidor-Reiss T, et al. Atypical centrioles during sexual reproduction. Front Cell Dev Biol. 2015;3(21).
Cavazza T, et al. Parental genome unification is highly erroneous in mammalian embryos. bioRxiv. 2020:2020.08.27.269779.
Schneider I, et al. Non-rodent mammalian zygotes assemble dual spindles despite the presence of paternal centrosomes. bioRxiv. 2020:2020.10.16.342154.
Griffin DK, Harton GL. Preimplantation genetic testing: recent advances in reproductive medicine: CRC Press; 2020.
Dupree JM. Insurance coverage for male infertility care in the United States. Asian J Androl. 2016;18(3):339–41.
Agarwal A, Parekh N, Panner Selvam MK, Henkel R, Shah R, Homa ST, et al. Male oxidative stress infertility (MOSI): proposed terminology and clinical practice guidelines for management of idiopathic male infertility. World J Mens Health. 2019;37(3):296–312.
Saalu LC. The incriminating role of reactive oxygen species in idiopathic male infertility: an evidence based evaluation. Pak J Biol Sci. 2010;13(9):413–22.
Alahmar AT. Role of oxidative stress in male infertility: an updated review. J Human Reprod Sci. 2019;12(1):4–18.
Jung JH, Seo JT. Empirical medical therapy in idiopathic male infertility: promise or panacea? Clin Exp Reprod Med. 2014;41(3):108–14.
Arafa M, et al. Efficacy of antioxidant supplementation on conventional and advanced sperm function tests in patients with idiopathic male infertility. Antioxidants (Basel, Switzerland). 2020;9(3):219.
Kathrins M. MOXI trial-is it time to stop routinely recommending antioxidant therapy to infertile men? Fertil Steril. 2020;113(3):542.
Steiner AZ, et al. The effect of antioxidants on male factor infertility: the males, antioxidants, and infertility (MOXI) randomized clinical trial. Fertil Steril. 2020;113(3):552–560.e3.
Ko EY, Siddiqi K, Brannigan RE, Sabanegh ES. Empirical medical therapy for idiopathic male infertility: a survey of the American Urological Association. J Urol. 2012;187(3):973–8.
Pregl Breznik B, Kovačič B, Vlaisavljević V. Are sperm DNA fragmentation, hyperactivation, and hyaluronan-binding ability predictive for fertilization and embryo development in in vitro fertilization and intracytoplasmic sperm injection? Fertil Steril. 2013;99(5):1233–41.
Choe SA, Tae JC, Shin MY, Kim HJ, Kim CH, Lee JY, et al. Application of sperm selection using hyaluronic acid binding in intracytoplasmic sperm injection cycles: a sibling oocyte study. J Korean Med Sci. 2012;27(12):1569–73.
Worrilow KC, Eid S, Woodhouse D, Perloe M, Smith S, Witmyer J, et al. Use of hyaluronan in the selection of sperm for intracytoplasmic sperm injection (ICSI): significant improvement in clinical outcomes—multicenter, double-blinded and randomized controlled trial. Hum Reprod. 2012;28(2):306–14.
Erberelli RF, Salgado RM, Pereira DH, Wolff P. Hyaluronan-binding system for sperm selection enhances pregnancy rates in ICSI cycles associated with male factor infertility. JBRA Assist Reprod. 2017;21(1):2–6.
Yildirim M, Duvan CI, Pekel A, Ayrim A, Kafali H. Can hyaluronan binding assay predict the outcome of intrauterine insemination in couples with unexplained or mild male factor infertility? J Reprod Infertil. 2015;16(1):18–23.
Natali A, Turek PJ. An assessment of new sperm tests for male infertility. Urology. 2011;77(5):1027–34.
Shukla KK, Mahdi AA, Rajender S. Apoptosis, spermatogenesis and male infertility. Front Biosci (Elite Ed). 2012;4:746–54.
Weng S-L, Taylor SL, Morshedi M, Schuffner A, Duran EH, Beebe S, et al. Caspase activity and apoptotic markers in ejaculated human sperm. Mol Hum Reprod. 2002;8(11):984–91.
Oosterhuis GJE, Mulder AB, Kalsbeek-Batenburg E, Lambalk CB, Schoemaker J, Vermes I. Measuring apoptosis in human spermatozoa: a biological assay for semen quality? Fertil Steril. 2000;74(2):245–50.
Amdani SN, Yeste M, Jones C, Coward K. Phospholipase C zeta (PLCζ) and male infertility: clinical update and topical developments. Adv Biol Regul. 2016;61:58–67.
Heytens E, Parrington J, Coward K, Young C, Lambrecht S, Yoon SY, et al. Reduced amounts and abnormal forms of phospholipase C zeta (PLCζ) in spermatozoa from infertile men. Hum Reprod. 2009;24(10):2417–28.
Kashir J, et al. A maternally inherited autosomal point mutation in human phospholipase C zeta (PLCζ) leads to male infertility. Hum Reprod. 2011;27(1):222–31.
Nomikos M, Kashir J, Swann K, Lai FA. Sperm PLCζ: from structure to Ca2+ oscillations, egg activation and therapeutic potential. FEBS Lett. 2013;587(22):3609–16.
Nomikos M, Swann K, Lai FA. Starting a new life: sperm PLC-zeta mobilizes the Ca2+ signal that induces egg activation and embryo development. BioEssays. 2012;34(2):126–34.
Yoon S-Y, Jellerette T, Salicioni AM, Lee HC, Yoo MS, Coward K, et al. Human sperm devoid of PLC, zeta 1 fail to induce Ca2+ release and are unable to initiate the first step of embryo development. J Clin Invest. 2008;118(11):3671–81.
Caballero-Campo P, Buffone MG, Benencia F, Conejo-García JR, Rinaudo PF, Gerton GL. A role for the chemokine receptor CCR6 in mammalian sperm motility and Chemotaxis. J Cell Physiol. 2014;229(1):68–78.
Chyra-Jach D, et al. The associations between infertility and antioxidants, proinflammatory cytokines, and chemokines. Oxidative Med Cell Longev. 2018;2018:8354747.
Duan Y-G, Wehry UP, Buhren BA, Schrumpf H, Oláh P, Bünemann E, et al. CCL20-CCR6 axis directs sperm–oocyte interaction and its dysregulation correlates/associates with male infertility‡. Biol Reprod. 2020;103:630–42.
Muciaccia B, Padula F, Vicini E, Gandini L, Lenzi A, Stefanini M. Beta-chemokine receptors 5 and 3 are expressed on the head region of human spermatozoon. FASEB J. 2005;19(14):2048–50.
Zandieh Z, Ashrafi M, Aflatoonian K, Aflatoonian R. Human sperm DNA damage has an effect on immunological interaction between spermatozoa and fallopian tube. Andrology. 2019;7(2):228–34.
Umezu K, Hara K, Hiradate Y, Numabe T, Tanemura K. Stromal cell-derived factor 1 regulates in vitro sperm migration towards the cumulus-oocyte complex in cattle. PLoS One. 2020;15(4):e0232536.
Colaco S, Sakkas D. Paternal factors contributing to embryo quality. J Assist Reprod Genet. 2018;35(11):1953–68.
Roldan ER. Assessments of sperm quality integrating morphology, swimming patterns, bioenergetics and cell signalling. Theriogenology. 2020;150:388–95.
Barratt CLR, Mansell S, Beaton C, Tardif S, Oxenham SK. Diagnostic tools in male infertility-the question of sperm dysfunction. Asian J Androl. 2011;13(1):53–8.
Hamilton BH, Jungheim E, McManus B, Pantano J. Health care access, costs, and treatment dynamics: evidence from in vitro fertilization. Am Econ Rev. 2018;108(12):3725–77.
Strasser MO, Dupree JM. Care delivery for male infertility: the present and future. Urol Clin. 2020;47(2):193–204.
Funding
We would like to thank the University of Toledo for sponsoring S.P. for this project through the Medical Student Research Program. This work was supported by Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) grant number R03 HD098314.
Author information
Authors and Affiliations
Corresponding author
Additional information
Glossary
• Anatomical causes—category within explained male infertility, any defect in or within the anatomy that affects mechanical processes that can lead to male infertility
• Asthenospermia—low motility
• Azoospermia—no sperm in semen
• Category—a broad group in which similar causes are classified together
• Cause—a diagnosable and treatable condition
• Explained male infertility—diagnosable and treatable cause of male factor infertility found, abnormal semen analysis, explained categories include anatomical, hormonal, genetic and environmental
• Extracellular sperm defect—subtype of idiopathic male infertility, an abnormality in the environment that sperm is suspended in (semen volume, viscosity, pH)
• Female infertility—infertility caused by female factors only
• Frequency of sperm defects—percentage of general sperm defects
• General sperm defect—subtype of idiopathic male infertility, an abnormality in the general characteristics of the sperm (count, shape, motility, viability)
• Genetics and environmental causes—category within explained male infertility, genetic abnormalities and/or external biological, chemical and other external factors intertwining to affect processes that can lead to male infertility
• Hormonal causes—category within explained male infertility, any hormonal imbalance leading to processes affecting sperm production, sexual desire, or any other hormonal process that can lead to male infertility
• Hyperspermia—high sperm count
• Idiopathic male infertility—category within male factor infertility, abnormal semen analysis but no demonstrable cause for that abnormality, two subtypes based on location are general sperm defects and extracellular sperm defects
• Infertility—inability to achieve pregnancy after 1 year of regular unprotected sexual intercourse
• Intracellular sperm analysis—a group of diagnostic tests that are able to detect intracellular sperm components such as chromosomes and centrioles
• Intracellular sperm defect—an abnormality inside of the sperm (DNA, RNA, centrioles, reactive oxygen species)
• Male infertility—a term that encompasses male factor infertility and unexplained male infertility
• Male factor infertility—infertility caused by male factors only, abnormal semen analysis
• Necrospermia—dead sperm in semen
• Oligospermia—low sperm count
• Prevalence of male infertility causes—percentage of causes of male infertility
• Teratospermia—abnormal sperm morphology (shape)
• Undetermined male infertility—infertile males with unknown causes, this class includes idiopathic male infertility and unexplained male infertility
• Unexplained couple infertility—no evidence of male or female infertility found
• Unexplained male infertility—males with unexplained couple infertility, normal semen analysis
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Semen analysis is limited and insufficient for evaluation of male infertility.
• One-fifth of infertile males have no identified cause for their infertility (undetermined male infertility).
• Intracellular sperm analysis can help resolve undetermined male infertility and improve treatment.
Supplementary Information
ESM 1
(DOCX 58 kb)
Rights and permissions
About this article
Cite this article
Pandruvada, S., Royfman, R., Shah, T.A. et al. Lack of trusted diagnostic tools for undetermined male infertility. J Assist Reprod Genet 38, 265–276 (2021). https://doi.org/10.1007/s10815-020-02037-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-020-02037-5