Abstract
Purpose
Intrafollicular fluid (IFF) melatonin plays a decisive role in maintaining granulosa cells’ DNA integrity and protects them against apoptosis. It reduces oxidative stress and improves the oocyte quality with a higher fertilization rate.
Method
This prospective study investigated the antioxidant property of IFF melatonin and its impact on IVF outcome parameters. We also explored the relative expression of five microRNAs (miR-663b, miR-320a, miR-766-3p, miR-132-3p, miR-16-5p) and levels of cell-free DNA (cfDNA) by real-time PCR in unexplained infertile patients. We collected 425 follicular fluid (FF) samples containing mature oocytes from 295 patients undergoing IVF.
Results
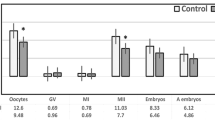
Patients were subgrouped based on IFF melatonin concentration (group A ≤ 30 pg/mL, group B > 70 to ≤ 110 pg/mL, group C > 111 to ≤ 385 pg/mL). Our results showed that patients with ≤ 30 pg/mL IFF melatonin levels have significantly higher oxidative stress markers, cfDNA levels, and lower relative expression of miR-663b, miR-320a, miR-766-3p, miR-132-3p, and miR-16-5p compared to other subgroups (p < 0.001). Similarly, they have a low fertilization rate and a reduced number of high-quality day 3 embryos.
Conclusion
Findings suggest that the therapeutic use of melatonin produces a considerable rise in the number of mature oocytes retrieved, fertilization rate, and good-quality embryo selection. Furthermore, miRNA signature enhances the quality of embryo selection, thus, may allow us to classify them as non-invasive biomarkers to identify good-quality embryos.



Similar content being viewed by others
Data availability
The data set used and analyzed during the current study is available from the corresponding author on reasonable request.
Abbreviations
- IVF:
-
in vitro fertilization
- IFF:
-
intrafollicular fluid
- AFC:
-
antral follicle count
- AMH:
-
anti-Müllerian hormone
- PCOS:
-
polycystic ovarian syndrome
- cfDNA:
-
cell-free DNA
- FF:
-
follicular fluid
- PGD:
-
preimplantation genetic diagnosis
- hESCs:
-
human endometrial stromal cells
- TAC:
-
total antioxidant capacity
- ROS:
-
reactive oxygen species
- SOD:
-
superoxide dismutase
- GSH:
-
glutathione
- miRNA:
-
microRNA
- ROC:
-
receiving operating characteristics
- E2 :
-
17β-estradiol
- BMI:
-
body mass index
- FSH:
-
follicular stimulating hormone
- LH:
-
luteinizing hormone
- TSH:
-
thyroid-stimulating hormone
- TVS:
-
transvaginal ultrasonography
- COS:
-
controlled ovarian stimulation
- ICSI:
-
intracytoplasmic sperm injection
- TBARS:
-
thiobarbituric acid reactive substances
- 8-OHdG:
-
8-hydroxy-2′-deoxyguanosine
- CI:
-
confidence interval
References
Turchi P. Prevalence, definition, and classification of infertility. In: Clinical management of male infertility, no. 1. Cham: Springer; 2015. pp. 5–11.
Gelbaya TA, Potdar N, Jeve YB, Nardo LG. Definition and epidemiology of unexplained infertility. Obstet Gynecol Surv. 2014;69(2):109–15.
Jana SK, Babu N, Chattopadhyay R, Chakravarty B, Chaudhury K. Upper control limit of reactive oxygen species in follicular fluid beyond which viable embryo formation is not favorable. Reprod Toxicol. 2010;29(4):447–51.
Oyawoye OA, Abdel-Gadir A, Garner A, Leonard AJ, Perrett C, Hardiman P. The interaction between follicular fluid total antioxidant capacity, infertility and early reproductive outcomes during in vitro fertilization. Redox Rep. 2009;14(5):205–13.
Espino J, Macedo M, Lozano G, Ortiz Á, Rodríguez C, Rodríguez AB, et al. Impact of melatonin supplementation in women with unexplained infertility undergoing fertility treatment. Antioxidants. 2019;8(9):338.
Becatti M, Fucci R, Mannucci A, Barygina V, Mugnaini M, Criscuoli L, et al. A biochemical approach to detect oxidative stress in infertile women undergoing assisted reproductive technology procedures. Int J Mol Sci. 2018;19(2):592.
Cadet J, Davies KJ. Oxidative DNA damage & repair: an introduction. Free Radic Biol Med. 2017;107:2–12.
Pauli A, Rinn JL, Schier AF. Non-coding RNAs as regulators of embryogenesis. Nat Rev Genet. 2011;12(2):136–49.
Martinez RM, Liang L, Racowsky C, Dioni L, Mansur A, Adir M, et al. Extracellular microRNAs profile in human follicular fluid and IVF outcomes. Sci Rep. 2018;8(1):1–10.
Machtinger R, Rodosthenous RS, Adir M, Mansour A, Racowsky C, Baccarelli AA, et al. Extracellular microRNAs in follicular fluid and their potential association with oocyte fertilization and embryo quality: an exploratory study. J Assist Reprod Genet. 2017;34(4):525–33.
Scalici E, Traver S, Mullet T, Molinari N, Ferrieres A, Brunet C, et al. Circulating microRNAs in follicular fluid, powerful tools to explore in vitro fertilization process. Sci Rep. 2016;6(1):1–10.
Santonocito M, Vento M, Guglielmino MR, Battaglia R, Wahlgren J, Ragusa M, et al. Molecular characterization of exosomes and their microRNA cargo in human follicular fluid: bioinformatic analysis reveals that exosomal microRNAs control pathways involved in follicular maturation. Fertil Steril. 2014;102(6):1751–61 e1751.
Montazerian M, Yasari F, Aghaalikhani N. Ovarian extracellular microRNAs as the potential non-invasive biomarkers: an update. Biomed Pharmacother. 2018;106:1633–40.
Qasemi M, Amidi F. Extracellular microRNA profiling in human follicular fluid: new biomarkers in female reproductive potential. J Assist Reprod Genet. 2020;37:1769–80.
Liang J, Wang S, Wang Z. Role of microRNAs in embryo implantation. Reprod Biol Endocrinol. 2017;15(1):90.
Capalbo A, Ubaldi FM, Cimadomo D, Noli L, Khalaf Y, Farcomeni A, et al. MicroRNAs in spent blastocyst culture medium are derived from trophectoderm cells and can be explored for human embryo reproductive competence assessment. Fertil Steril. 2016;105(1):225–35 e223.
Feng R, Sang Q, Zhu Y, Fu W, Liu M, Xu Y, et al. MiRNA-320 in the human follicular fluid is associated with embryo quality in vivo and affects mouse embryonic development in vitro. Sci Rep. 2015;5:8689.
Abu-Halima M, Khaizaran ZA, Ayesh BM, Fischer U, Khaizaran SA, Al-Battah F, et al. MicroRNAs in combined spent culture media and sperm are associated with embryo quality and pregnancy outcome. Fertil Steril. 2020;133(5):970-980.e2.
Kropp J, Khatib H. Characterization of microRNA in bovine in vitro culture media associated with embryo quality and development. J Dairy Sci. 2015;98(9):6552–63.
Sang Q, Yao Z, Wang H, Feng R, Wang H, Zhao X, et al. Identification of microRNAs in human follicular fluid: characterization of microRNAs that govern steroidogenesis in vitro and are associated with polycystic ovary syndrome in vivo. J Clin Endocrinol Metab. 2013;98(7):3068–79.
Srinivasan V, Singh J, Pandi-Perumal SR, Brown GM, Spence DW, Cardinali DP. Jet lag, circadian rhythm sleep disturbances, and depression: the role of melatonin and its analogs. Adv Ther. 2010;27(11):796–813.
Cajochen C, Kräuchi K, Wirz-Justice A. Role of melatonin in the regulation of human circadian rhythms and sleep. J Neuroendocrinol. 2003;15(4):432–7.
Hadi A, Ghaedi E, Moradi S, Pourmasoumi M, Ghavami A, Kafeshani M. Effects of melatonin supplementation on blood pressure: a systematic review and meta-analysis of randomized controlled trials. Horm Metab Res. 2019;51(03):157–64.
Najafi M, Shirazi A, Motevaseli E, Geraily G, Norouzi F, Heidari M, et al. The melatonin immunomodulatory actions in radiotherapy. Biophys Rev. 2017;9(2):139–48.
Gaspar do Amaral F, Cipolla-Neto J. A brief review about melatonin, a pineal hormone. Arch Endocrinol Metab. 2018;62(4):472–9.
Sanchez-Hidalgo M, Alarcon de la Lastra C, Carrascosa-Salmoral MP, Naranjo MC, Gomez-Corvera A, Caballero B, et al. Age-related changes in melatonin synthesis in rat extrapineal tissues. Exp Gerontol. 2009;44(5):328–34.
Bodis J, Hartmann G, Tinneberg H-R, Török A, Hanf V, Papenfuss F, et al. Relationship between the monoamine, progesterone and estradiol content in follicular fluid of preovulatory graafian follicles after superovulation treatment. Gynecol Obstet Investig. 1993;35(4):232–5.
Itoh MT, Ishizuka B, Kuribayashi Y, Amemiya A, Sumi Y. Melatonin, its precursors, and synthesizing enzyme activities in the human ovary. Mol Hum Reprod. 1999;5(5):402–8.
Tamura H, Tanabe M, Jozaki M, Taketani T, Sugino N. Antioxidative action of melatonin and reproduction. Glycative Stress Research. 2019;6(3):192–7.
Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J Pineal Res. 2007;42(1):28–42.
Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martín V, et al. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res. 2004;36(1):1–9.
Tamura H, Nakamura Y, Korkmaz A, Manchester LC, Tan D-X, Sugino N, et al. Melatonin and the ovary: physiological and pathophysiological implications. Fertil Steril. 2009;92(1):328–43.
Khan HL, Bhatti S, Khan YL, Abbas S, Munir Z, Sherwani IARK, et al. Cell-free nucleic acids and melatonin levels in human follicular fluid predict embryo quality in patients undergoing in-vitro fertilization treatment. J Gynecol Obstet Hum Reprod. 2020;49(1):101624.
Tanabe M, Tamura H, Taketani T, Okada M, Lee L, Tamura I, et al. Melatonin protects the integrity of granulosa cells by reducing oxidative stress in nuclei, mitochondria, and plasma membranes in mice. J Reprod Dev. 2015;61:35–41.
Tamura H, Takasaki A, Taketani T, Tanabe M, Kizuka F, Lee L, et al. Melatonin as a free radical scavenger in the ovarian follicle. Endocr J. 2013;60:1–13.
Nakamura Y, Tamura H, Takayama H, Kato H. Increased endogenous level of melatonin in preovulatory human follicles does not directly influence progesterone production. Fertil Steril. 2003;80(4):1012–6.
Ishizuka B, Kuribayashi Y, Murai K, Amemiya A, Itoh MT. The effect of melatonin on in vitro fertilization and embryo development in mice. J Pineal Res. 2000;28(1):48–51.
Rodriguez-Osorio N, Kim I, Wang H, Kaya A, Memili E. Melatonin increases cleavage rate of porcine preimplantation embryos in vitro. J Pineal Res. 2007;43(3):283–8.
Asgari Z, Ghasemian F, Ramezani M, Bahadori MH. The effect of melatonin on the developmental potential and implantation rate of mouse embryos. Cell J. 2012;14(3):203.
Kim MK, Park EA, Kim HJ, Choi WY, Cho JH, Lee WS, et al. Does supplementation of in-vitro culture medium with melatonin improve IVF outcome in PCOS? Reprod BioMed Online. 2013;26(1):22–9.
Matsunaga R, Watanabe S, Mita W, Miura M, Kobayashi Y, Yamanaka N, et al. Effect of melatonin on developmental competence of denuded human oocytes during in vitro maturation. Fertil Steril. 2017;108(3):e145–6.
Tamura H, Takasaki A, Miwa I, Taniguchi K, Maekawa R, Asada H, et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res. 2008;44(3):280–7.
Nishihara T, Hashimoto S, Ito K, Nakaoka Y, Matsumoto K, Hosoi Y, et al. Oral melatonin supplementation improves oocyte and embryo quality in women undergoing in vitro fertilization-embryo transfer. Gynecol Endocrinol. 2014;30(5):359–62.
Tamura H, Jozaki M, Tanabe M, Shirafuta Y, Mihara Y, Shinagawa M, et al. Importance of melatonin in assisted reproductive technology and ovarian aging. Int J Mol Sci. 2020;21(3):1135.
Unfer V, Raffone E, Rizzo P, Buffo S. Effect of a supplementation with myo-inositol plus melatonin on oocyte quality in women who failed to conceive in previous in vitro fertilization cycles for poor oocyte quality: a prospective, longitudinal, cohort study. Gynecol Endocrinol. 2011;27(11):857–61.
Jahnke G, Marr M, Myers C, Wilson R, Travlos G, Price C. Maternal and developmental toxicity evaluation of melatonin administered orally to pregnant Sprague-Dawley rats. Toxicol Sci. 1999;50(2):271–9.
Khaksar M, Oryan A, Sayyari M, Rezabakhsh A, Rahbarghazi R. Protective effects of melatonin on long-term administration of fluoxetine in rats. Exp Toxicol Pathol. 2017;69(8):564–74.
Sugden D. Psychopharmacological effects of melatonin in mouse and rat. J Pharmacol Exp Ther. 1983;227(3):587–91.
Andersen LPH, Gögenur I, Rosenberg J, Reiter RJ. The safety of melatonin in humans. Clin Drug Investig. 2016;36(3):169–75.
Bejarano I, Monllor F, Marchena AM, Ortiz A, Lozano G, Jiménez MI, et al. Exogenous melatonin supplementation prevents oxidative stress-evoked DNA damage in human spermatozoa. J Pineal Res. 2014;57(3):333–9.
World Health Organization. WHO laboratory manual for the examination and processing of human semen, 5th edn. Geneva: World Health Organization; 2010. pp. 1–286.
Vandekerckhove F, Vansteelandt S, Gerris J, De Sutter P. Follicle measurements using sonography-based automated volume count accurately predict the yield of mature oocytes in in vitro fertilization/intracytoplasmic sperm injection cycles. Gynecol Obstet Investig. 2013;76(2):107–12.
Hernández J, Rodríguez-Fuentes A, Puopolo M, Palumbo A. Follicular volume predicts oocyte maturity: a prospective cohort study using three-dimensional ultrasound and SonoAVC. Reprod Sci. 2016;23(12):1639–43.
Lazzaroni-Tealdi E, Barad DH, Albertini DF, Yu Y, Kushnir VA, Russell H, et al. Oocyte scoring enhances embryo-scoring in predicting pregnancy chances with IVF where it counts most. PLoS One. 2015;10(12):e0143632.
Dimopoulou M, Anifandis G, Messini C, Dafopoulos K, Kouris S, Sotiriou S, et al. Follicular fluid oocyte/cumulusfree DNA concentrations as a potential biomolecular marker of embryo quality and IVF outcome. Biomed Res Int. 2014;2014:289306.
Munakata Y, Shirasuna K, Kuwayama T, Iwata H. Cell-free DNA in medium is associated with the maturation ability of in vitro cultured oocytes. J Reprod Dev. 2019;65 (2):171–5.
Attaran M, Pasqualotto E, Falcone T, Goldberg JM, Miller KF, Agarwal A, et al. The effect of follicular fluid reactive oxygen species on the outcome of in vitro fertilization. Int J Fertil Womens Med. 2000;45(5):314–20.
Rice-Evans CA, Diplock AT, Symons MR. Techniques in free radical research. Lab Techn Biochem Mol Biol. 1991;22:1–278.
Sarhan D, El Mazny A, Taha T, Aziz A, Azmy O, Fakhry D, et al. Estradiol and luteinizing hormone concentrations in the follicular aspirate during ovum pickup as predictors of in vitro fertilization (IVF) outcome. Middle East Fertil Soc J. 2017;22(1):27–32.
Zheng P, Si W, Bavister BD, Yang J, Ding C, Ji W. 17β-estradiol and progesterone improve in-vitro cytoplasmic maturation of oocytes from unstimulated prepubertal and adult rhesus monkeys. Hum Reprod. 2003;18(10):2137–44.
Polak G, Rola R, Gogacz M, Kozioł-Montewka M, Kotarski J. Malonyldialdehyde and total antioxidant status in the peritoneal fluid of infertile women. Ginekol Pol. 1999;70(3):135–40.
Fernando S, Osianlis T, Vollenhoven B, Wallace E, Rombauts L. A pilot double-blind randomised placebo-controlled dose–response trial assessing the effects of melatonin on infertility treatment (MIART): study protocol. BMJ Open. 2014;4(8). https://doi.org/10.1136/bmjopen-2014-005986.
Ménézo Y, Dale B, Cohen M. DNA damage and repair in human oocytes and embryos: a review. Zygote. 2010;18(4):357–65.
Antolín I, Rodríguez C, Sáinz RM, Mayo JC, Uría H, Kotler ML, et al. Neurohormone melatonin prevents cell damage: effect on gene expression for antioxidant enzymes. FASEB J. 1996;10(8):882–90.
Mayo J, Sainz R, Antolin I, Herrera F, Martin V, Rodriguez C. Melatonin regulation of antioxidant enzyme gene expression. Cell Mol Life Sci. 2002;59(10):1706–13.
Seino T, Saito H, Kaneko T, Takahashi T, Kawachiya S, Kurachi H. Eight-hydroxy-2′-deoxyguanosine in granulosa cells is correlated with the quality of oocytes and embryos in an in vitro fertilization-embryo transfer program. Fertil Steril. 2002;77(6):1184–90.
Xu G, Zhao J, Liu H, Wang J, Lu W. Melatonin inhibits apoptosis and oxidative stress of mouse leydig cells via a SIRT1-dependent mechanism. Molecules. 2019;24(17):3084.
Liu R, Fu A, Hoffman AE, Zheng T, Zhu Y. Melatonin enhances DNA repair capacity possibly by affecting genes involved in DNA damage responsive pathways. BMC Cell Biol. 2013;14(1):1–8.
Berkhout RP, Keijser R, Repping S, Lambalk CB, Afink GB, Mastenbroek S, et al. High-quality human preimplantation embryos stimulate endometrial stromal cell migration via secretion of microRNA hsa-miR-320a. bioRxiv. 2020;21(91):32–6.
Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2011;75(1):50–83.
Wang D, Luo Y, Wang G, Yang Q. Circular RNA expression profiles and bioinformatics analysis in ovarian endometriosis. Mol Genet Genomic Med. 2019;7(7):e00756.
Su S-C, Reiter RJ, Hsiao H-Y, Chung W-H, Yang S-F. Functional interaction between melatonin signaling and noncoding RNAs. Trends Endocrinol Metab. 2018;29(6):435–45.
Diez-Fraile A, Lammens T, Tilleman K, Witkowski W, Verhasselt B, De Sutter P, et al. Age-associated differential microRNA levels in human follicular fluid reveal pathways potentially determining fertility and success of in vitro fertilization. Hum Fertil. 2014;17(2):90–8.
Salas-Huetos A, James ER, Aston KI, Jenkins TG, Carrell DT, Yeste M. The expression of miRNAs in human ovaries, oocytes, extracellular vesicles, and early embryos: a systematic review. Cells. 2019;8(12):1564.
Liang L-F, Qi S-T, Xian Y-X, Huang L, Sun X-F, Wang W-H. Protective effect of antioxidants on the pre-maturation aging of mouse oocytes. Sci Rep. 2017;7(1):1–10.
Reza AMMT, Choi YJ, Han SG, Song H, Park C, Hong K, et al. Roles of microRNAs in mammalian reproduction: from the commitment of germ cells to peri-implantation embryos. Biol Rev. 2019;94(2):415–38.
Ragusa M, Barbagallo D, Chioccarelli T, Manfrevola F, Cobellis G, Di Pietro C, et al. CircNAPEPLD is expressed in human and murine spermatozoa and physically interacts with oocyte miRNAs. RNA Biol. 2019;16(9):1237–48.
Fu J, Qu R-g, Zhang Y-j, Gu R-h, Li X, Sun Y-j, et al. Screening of miRNAs in human follicular fluid reveals an inverse relationship between microRNA-663b expression and blastocyst formation. Reprod BioMed Online. 2018;37(1):25–32.
Acknowledgments
We acknowledge the research initiative and gratefully thank Professor Dr. Rashid Latif Khan, Professor of Emeritus in Obstetrics and Gynecology, for manuscript editing. The study is conducted under the infertility research center of Hameed Latif Hospital.
Author information
Authors and Affiliations
Contributions
HLK and YLK: reviewing and editing, software; SB: conceptualization, methodology, software, data curation, project administration, writing—original draft preparation, supervision, writing—reviewing and editing; SA: formal analysis, validation, methodology, investigation, writing—reviewing and editing; CK: writing—reviewing and editing; SQ: data curation, writing—original draft preparation; ZH: reviewing and editing, methodology, software; NZT: writing—reviewing and editing; HHY: software, validation, methodology.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
The study was approved by our Institutional Ethical Committee (IEC). Informed consent was obtained from all subjects before the research and publishing of the results of the investigation.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 26 kb).
Figure 1S
Relationship between cfDNA levels and melatonin concentration in individual FF samples. (PNG 194 kb).
Figure 2S
Pearson’s correlation between IFF melatonin concentration and different biomarkers of oxidative balance. (A) Total antioxidant capacity (TAC) (B) Reactive oxygen species (ROS) (C) Thiobarbituric acid reactive substances (TBARS) and (D) 8-hydroxy-2′-deoxyguanosine (8-OHdG) concentrations. (PNG 220 kb).
ESM 1
(PNG 190 kb).
ESM 2
(PNG 229 kb).
ESM 3
(PNG 204 kb).
Figure 3S
Predicted pathway analysis heat map for all miRNAs in detail. Red color indicates high expression and lower p values. Yellow color indicates intermediate expression. (PNG 974 kb).
Rights and permissions
About this article
Cite this article
Khan, H.L., Bhatti, S., Abbas, S. et al. Melatonin levels and microRNA (miRNA) relative expression profile in the follicular ambient microenvironment in patients undergoing in vitro fertilization process. J Assist Reprod Genet 38, 443–459 (2021). https://doi.org/10.1007/s10815-020-02010-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-020-02010-2