Abstract
Purpose
To evaluate whether adjusting timing of modified natural cycle frozen embryo transfer (mNC-FET) 1 day earlier in the setting of a spontaneous LH surge has an impact on pregnancy outcomes.
Methods
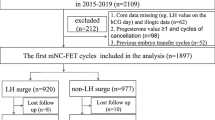
This retrospective cohort study evaluated all mNC-FET with euploid blastocysts from May 1, 2016 to March 30, 2019, at a single academic institution. Standard protocol for mNC-FET included ultrasound monitoring and hCG trigger when the dominant follicle and endometrial lining were appropriately developed. Patients had serum LH, estradiol, and progesterone checked on day of trigger. If LH was ≥ 20 mIU/mL, trigger was given that day and FET was performed 6 days after surge (LH/HCG+6), with the intent of transferring 5 days after ovulation. If LH was < 20 mIU/mL, FET was performed 7 days after trigger (hCG+7). Primary outcomes included clinical pregnancy and live birth rates. To account for correlation between cycles, a generalized estimating equation (GEE) method for multivariable logistic regression was used.
Results
Four hundred fifty-three mNC-FET cycles met inclusion criteria, of which 205 were in the LH/HCG+6 group and 248 were in the HCG+7 group. The overall clinical pregnancy rate was 64% and clinical miscarriage rate was 4.8%, with similar rates between the two groups. The overall live birth rate was 60.9% (61.0% in LH/HCG+6 group and 60.9% in HCG+7 group). After implementing GEE, the odds of CP (aOR 0.97, 95% CI [0.65–1.45], p = 0.88) and LB (aOR 0.98, 95% CI [0.67–1.45], p = 0.93) were similar in both groups.
Conclusions
In our study cohort, mNC-FET based on LH/HCG+6 versus HCG+7 had similar pregnancy outcomes.

Similar content being viewed by others
References
Centers for Disease Control and Prevention, American Society for Reproductive Medicine, Society for Assisted Reproductive Technology. Assisted reproductive technology national summary report. Atlanta: US Dept of Health and Human Services; 2014. p. 2016.
Centers for Disease Control and Prevention, American Society for Reproductive Medicine, Society for Assisted Reproductive Technology. Assisted reproductive technology national summary report. Atlanta: US Dept of Health and Human Services; 2015. p. 2017.
Centers for Disease Control and Prevention, American Society for Reproductive Medicine, Society for Assisted Reproductive Technology. Assisted reproductive technology national summary report. Atlanta: US Dept of Health and Human Services; 2016. p. 2018.
Baker VL, Iko I, Segars J. Is a frozen embryo transfer in a programmed cycle really the best option? J Assist Reprod Genet. 2019;36:935–7.
Tabibzadeh S. Molecular control of the implantation window. Hum Reprod Update. 1998;4:465–71.
Fatemi HM, Kyrou D, Bourgain C, Van den Abbeel E, Griesinger G, Devroey P. Cryopreserved-thawed human embryo transfer: spontaneous natural cycle is superior to human chorionic gonadotropin-induced natural cycle. Fertil Steril. 2010;94:2054–8.
Groenewoud ER, Kollen BJ, Macklon NS, Cohlen BJ. Spontaneous LH surges prior to HCG administration in unstimulated-cycle frozen-thawed embryo transfer do not influence pregnancy rates. Reprod BioMed Online. 2012;24:191–6.
Irani M, Robles A, Gunnala V, Reichman D, Rosenwaks Z. Optimal parameters for determining the LH surge in natural cycle frozen-thawed embryo transfers. J Ovarian Res. 2017;10:70.
Austin P. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Communications in Statistics-Simulation and Computation. 2009;38:1228–34.
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing 2019, Vienna, Austria. https://www.R-project.org/. Accessed 1 Aug 2020.
Højsgaard S, Halekoh U, Yan J. The R Package geepack for generalized estimating equations. J Stat Softw. 2006;15:1–11.
Yan J, Fine J. Estimating equations for association structures. Stat Med. 2004;23:859–80.
Yan J. Geepack: yet another package for generalized estimating equations. R News. 2002;2:12–4.
Mounce G, McVeigh E, Turner K, Child TJ. Randomized, controlled pilot trial of natural versus hormone replacement therapy cycles in frozen embryo replacement in vitro fertilization. Fertil Steril. 2015;104:915–20.
Cerrillo M, Herrero L, Guillén A, Mayoral M, García-Velasco JA. Impact of endometrial preparation protocols for frozen embryo transfer on live birth rates. Rambam Maimonides Med J. 2017;8.
Kalem Z, Kalem M, Bakırarar B, Kent E, Gurgan T. Natural cycle versus hormone replacement therapy cycle in frozen-thawed embryo transfer. Saudi Med J. 2018;39:1102–8.
Lathi RB, Chi YY, Liu J, Saravanabavanandhan B, Hegde A, Baker VL. Frozen blastocyst embryo transfer using a supplemented natural cycle protocol has a similar live birth rate compared to a programmed cycle protocol. J Assist Reprod Genet. 2015;32:1057–62.
Devine K, Richter KS, Widra EA, McKeeby JL. Vitrified blastocyst transfer cycles with the use of only vaginal progesterone replacement with Endometrin have inferior ongoing pregnancy rates: results from the planned interim analysis of a three-arm randomized controlled noninferiority trial. Fertil Steril. 2018;109:266–75.
Ginström Ernstad E, Wennerholm UB, Khatibi A, Petzold M, Bergh C. Neonatal and maternal outcome after frozen embryo transfer: Increased risks in programmed cycles. Am J Obstet Gynecol. 2019;221:126.e1–126.e18.
von Versen-Höynck F, Schaub AM, Chi YY, Chiu KH, Liu J, Lingis M, et al. Increased preeclampsia risk and reduced aortic compliance with in vitro fertilization cycles in the absence of a corpus luteum. Hypertension. 2019;73:640–9.
Bartels CB, Ditrio L, Grow DR, O'Sullivan DM, Benadiva CA, Engmann L, et al. The window is wide: flexible timing for vitrified-warmed embryo transfer in natural cycles. Reprod BioMed Online. 2019;39:241–8.
van der Linden M, Buckingham K, Farquhar C, Kremer JA, Metwally M. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst Rev. 2015;2015:CD009154.
Reichman DE, Stewart CR, Rosenwaks Z. Natural frozen embryo transfer with hCG booster leads to improved cycle outcomes: a retrospective cohort study. J Assist Reprod Genet. 2020;37:1177–82.
Polyzos NP, Messini CI, Papanikolaou EG, Mauri D, Tzioras S, Badawy A, et al. Vaginal progesterone gel for luteal phase support in IVF/ICSI cycles: a meta-analysis. Fertil Steril. 2010;94:2083–7.
Shiba R, Kinutani M, Okano S, Kawano R, Kikkawa Y. Efficacy of four vaginal progesterones for luteal phase support in frozen-thawed embryo transfer cycles: a randomized clinical trial. Reprod Med Biol. 2020;19:42–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
J. K. Johal and B. Bavan are co first authors.
Rights and permissions
About this article
Cite this article
Johal, J.K., Bavan, B., Zhang, W. et al. The impact of timing modified natural cycle frozen embryo transfer based on spontaneous luteinizing hormone surge. J Assist Reprod Genet 38, 219–225 (2021). https://doi.org/10.1007/s10815-020-01994-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-020-01994-1