Abstract
Purpose
Microgravity has severe effects on cellular and molecular structures as well as on metabolic interactions. The aim of this study is to investigate the effects of microgravity (μg) exposure on human frozen sperm samples.
Methods
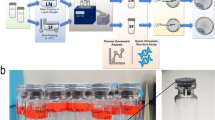
Sibling samples from 15 normozoospermic healthy donors were frozen using glycerol as cryoprotectant and analyzed under microgravity and ground conditions. Microgravity was obtained by parabolic flights using a CAP10B plane. The plane executed 20 parabolic maneuvers with a mean of 8.5 s of microgravity for each parabola.
Results
Frozen sperm samples preserved in cryostraws and stored in a secure and specific nitrogen vapor cryoshipper do not suffer significant alterations after μg exposure. Comparing the study group (μg) and the control group (1 g), similar results were obtained in the main parameters studied: sperm motility (M/ml) 13.72 ± 12.57 vs 13.03 ± 12.13 (− 0.69 95% CI [− 2.9; 1.52]), progressive a + b sperm motility (%) 21.83 ± 11.69 vs 22.54 ± 12.83 (0.03 95% CI [− 0.08; 0.15]), sperm vitality (%) 46.42 ± 10.81 vs 44.62 ± 9.34 (− 0.04 95% CI [− 0.13; 0.05]), morphologically normal spermatozoa (%) 7.03 ± 2.61 vs 8.09 ± 3.61 (0.12 95% CI [0.01; 0.24]), DNA sperm fragmentation by SCD (%) 13.33 ± 5.12 vs 13.88 ± 6.14 (0.03 95% CI [− 0.09; 0.16]), and apoptotic spermatozoa by MACS (%) 15.47 ± 15.04 vs 23.80 ± 23.63 (− 0.20 95% CI [− 0.66; 1.05]).
Conclusion
The lack of differences obtained between frozen samples exposed to μg and those maintained in ground conditions provides the possibility of considering the safe transport of human male gametes to space. Nevertheless, further research is needed to validate the results and to consider the possibility of creating a human sperm bank outside the Earth.
Trial registration number
ClinicalTrials.gov: NCT03760783
Similar content being viewed by others
Data availability
All relevant data are within the paper.
References
Clément G. Fundamentals of space medicine. 1st ed. Kluwer; 2003. p. 4.
Vaquer S, Cuyàs E, Rabadan A, González A, Fenollosa F, de la Torre R. Active transmembrane drug transport in microgravity: a validation study using an ABC transporter model. F1000Research. 2014;3:201.
Pietsch J, Bauer J, Egli M, Infanger M, Wise P, Ulbrich C, et al. The effects of weightlessness on the human organism and mammalian cells. Curr Mol Med. 2011;11:350–64.
Narici M, de Boer MD. Disuse of musculo-skeletal system in space and on earth. Eur J Appl Physiol. 2011;111:403–20.
Mandsager KT, Robertson D, Diedrich A. The function of the autonomic nervous system during space flight. Clin Auton Res. 2015;25:141–51.
Macho L, Kvetnansky R, Fickova M, Popova IA, Grigoriev A. Effects of exposure to space flight on endocrine regulations in experimental animals. Endocr Regul. 2001;35:101–14.
Osborne J, Alonsopérez MV, Ferrer D, Goswami N, González DV, Moser M, et al. Effect of mental arithmetic on heart rate responses during parabolic flights: the Barcelona zero-G challenge. Microgravity Sci Technol. 2014;26:11–6.
Jennings R, Baker E. Gynecological and reproductive issues for women in space: a review. Obstet Gynecol Surv. 2000;55:109–16.
Serova LV, Denisova LA, Lavrova EA, Makeyeva VF, Natochin YV, Pustynnikova AM, Shakhmatova EI. Parameters of the reproductive function of the mammals: Fetal and placental characteristics. In: OG Gazenko editors. Ontogenesis of mammals in microgravity. NASA TM-103978, Washington DC. 1993. pp. 35–6.
Ronca A. Mammalian development in space. In: Marty H-J, editor. Developmental Biology Research in Space. Elsevier Science; 2003. p. 217–51.
Pellegrini M, Di Siena S, Claps G, Di Cesare S, Dolci S, Rossi P, et al. Microgravity promotes differentiation and meiotic entry of postnatal mouse male germ cells. PLoS One. 2010;5(2):e9064. https://doi.org/10.1371/journal.pone.0009064.
Morabito C, Guarnieri S, Catizone A, Schiraldi C, Ricci G, Mariggio MA. Transient increases in intracellular calcium and reactive oxygen species levels in TCam-2 cells exposed to microgravity. Sci Rep. 2017;7:15648.
Shinde V, Brungs S, Henry M, Wegener L, Nemade H, Rotshteyn T, et al. Simulated microgravity modulates differentiation processes of embryonic stem cells. Cell Physiol Biochem. 2016;38:1483–99.
Nowacki D, Klinger F, Mazur G, De Felici M. Effects of culture in simulated microgravity on the development of mouse embryonic testes. Adv Clin Exp Med. 2015;24:769–74.
Tash JS, Johnson DC, Enders GC. Long term (6 wk) hind limb suspension inhibits spermatogenesis in adult male rats. J Appl Physiol. 2002;92:1191–8.
Zhang X, Li L, Bai Y, Shi R, Wei H, Zhang S. Mouse undifferentiated spermatogonial stem cells cultured as aggregates under simulated microgravity. Andrologia. 2014;46:1013–21.
Engelmann U, Krassnigg F, Schill WB. Sperm motility under conditions of weightlessness. J Androl. 1992;13:433–6.
Tash JS, Bracho GE. Microgravity alters protein phosphorylation changes during initiation of sea urchin sperm motility. FASEB J. 1999;13:S43–54.
Kamiya H, Sasaki S, Ikeuchi T, Umemoto Y, Tatsura H, Hayashi Y, et al. Effect of simulated microgravity on testosterone and sperm motility in mice. J Androl. 2003;24:885–90.
Ikeuchi T, Sasaki S, Umemoto Y, Kubota Y, Kubota H, Kaneko T, et al. Human sperm motility in a microgravity environment. Reprod Med Biol. 2005;4:161–7.
Wu C, Guo X, Wang F, Li X, X Cindy T, Li L, et al. Simulated microgravity compromises mouse oocyte maturation by disrupting meiotic spindle organization and inducing cytoplasmic blebbing. PLoS One. 2011;6(7):e22214. https://doi.org/10.1371/journal.pone.0022214.
Lin SC, Gou GH, Hsia CW, Ho CW, Huang KL, Wu YF, et al. Simulated microgravity disrupts cytoskeleton organization and increases apoptosis of rat neural crest stem cells via upregulating CXCR4 expression and RhoA-ROCK1-p38MAPK-p53 signaling. Stem Cells Dev. 2016;25:1172–93.
Barjaktarović Z, Nordheim A, Lamkemeyer T, Fladere C, Madlung J, Hampp R. Time-course of changes in amounts of specific proteins upon exposure to hyper-g, 2-D clinorotation, and 3-D random positioning of Arabidopsis cell cultures. J Exp Bot. 2007;58:4357–63.
Nishikawa M, Ohgushi H, Tamai N, Osuga K, Uemura M, Yoshikawa H, et al. The effect of simulated microgravity by three-dimensional clinostat on bone tissue engineering. Cell Transplant. 2005;14:829–35.
Kufner E, Blum J, Callens N, Eigenbrod C, Koudelka O, Orr A, et al. ESA’s drop tower utilization activities 2000 to 2011. Microgravity Sci Technol. 2011;23:409–25.
Dannenberg K. Swedish space activities - an overview with focus on balloons and rockets. In: Proceedings of the 200th ESA Symposium on European rocket and balloon programmes and related research. ESA Special publications. 2011. pp 33–5.
Pletser V. Short duration microgravity experiments in physical and life sciences during parabolic flights: the first 30 ESA campaigns. Acta Astronaut. 2004;55:829–54.
Callens N, Ventura-Traveset J, De Lophem TL, Lopez de Echazarreta C, Pletser V, Van Loon J. ESA Parabolic flights, drop tower and centrifuge opportunities for university students. Microgravity Sci Technol. 2011;23:181–9.
Pletser V, Winter J, Bret-Dibat T, Friedrich U, Clervoy JF, Gharib T, et al. The first joint European partial-G parabolic flight campaign at Moon and Mars gravity levels for science and exploration. Microgravity Sci Technol. 2012;24:383–95.
Pletser V, Rouquette S, Friedrich U, Clervoy J, Gharib T, Gai F, et al. European parabolic flight campaigns with Airbus zero-g: looking back at the A300 and looking forward to the A310. Adv Space Res. 2015;56:1003–13.
Brigos M, Perez-Poch A, Alpiste F, Torner J. Parabolic flights with single-engine aerobatic aircraft: flight profile and a computer simulator for its optimization. Microgravity Sci Technol. 2014;26:229–39.
Clément G, Allawey H, Demel M, Golemis A, Kindrat A, Melinyshyn A, et al. Long duration spaceflight increases depth ambiguity of reversible perspective figures. PLoS One. 2015;10(7):e0132317. https://doi.org/10.1371/journal.pone.0132317.
Schuster A, Boccia V, Perez-Poch A, Gonzalez DV. Estimation of relative distance between two objects in microgravity conditions during parabolic flight. Proceedings of the Elgra Symposium and general assembly. Elgra news. 2015;31:120.
Perez-Poch A, Ventura D, Lopez D. Hypogravity research and educational parabolic flight activities conducted in Barcelona: a new hub of innovation in Europe. Microgravity Sci Technol. 2016;28:603–9.
World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th ed. Switzerland: World Health Organization; 2010. ISBN 978 92 4 154778 9
Polge C. Low-temperature storage of mammalian spermatozoa. Proc R Soc Lond B Biol Sci. 1957;147:498–508.
Evenson D, Wixon R. Meta-analysis of sperm DNA fragmentation using the sperm chromatin structure assay. Reprod BioMed Online. 2006;12:466–72.
Kamal K, Herranz R, van Loon JJWA, Medina FJ. Simulated microgravity, Mars gravity, and 2g hypergravity affect cell cycle regulation, ribosome biogenesis, and epigenetics in Arabidopsis cell cultures. Sci Rep. 2018;8:6424.
Grimm D, Egli M, Krüger M, Riwaldt S, Corydon TJ, Kopp S, et al. Tissue engineering under microgravity conditions -use of stem cells and specialized cells. Stem Cells Dev. 2018;27:787–804.
Wakayama T, Yanagimachi R. Development of normal mice from oocytes injected with freeze-dried spermatozoa. Nat Biotechnol. 1998;16:639–41.
Wakayama S, Kamada Y, Kohda T, Suzuki H, Shimazu T, Tada M, et al. Healthy offspring from freeze-dried mouse spermatozoa held on the International Space Station for 9 months. PNAS. 2017;23:5988–93.
Gianaroli L, Magli MC, Stanghellini I, Crippa A, Crivello AM, Pescatori ES, et al. DNA integrity is maintained after freeze-drying of human spermatozoa. Fertil Steril. 2012;5:1067–73.
Isachenko E, Isachenko V, Katkov II, Dessole S, Nawroth F. Vitrification of mammalian spermatozoa in the absence of cryoprotectants: from past practical difficulties to present success. Reprod BioMed Online. 2003;10:191–200.
Isachenko V, Isachenko E, Montag M, Zaeva V, Krivokharchenko I, Nawroth F, et al. Clean technique for cryoprotectant-free vitrification of human spermatozoa. Reprod BioMed Online. 2005;10:350–4.
Li HY, Zhang H, Miao GY, Xie Y, Sun C, Di CX, et al. Simulated microgravity conditions and carbon ion irradiation induce spermatogenic cell apoptosis and sperm damage. Biomed Environ Sci. 2013;26:726–34.
Yatagai F, Ishioka N. Are biological effects of space radiation really altered under the microgravity environment? Life Sci Space Res. 2014;3:76–89.
Acknowledgments
The authors thank Ignacio Rodriguez for his support in the statistical analysis.
Code availability
Not applicable.
Funding
This work was performed under the auspices of Càtedra d’Investigació en Obstetrícia y Ginecologia of the Department of Obstetrics, Gynaecology and Reproduction, Dexeus Women’s Health and the Universitat Autònoma de Barcelona. The study was supported by a research grant from “Fundación Dexeus Mujer 2019” in the area of Basic Science (Reproductive Medicine).
Author information
Authors and Affiliations
Contributions
M. Boada and A. Perez-Poch conceived the study; D.V. González conducted the parabolic flights; M. Ballester and S. García-Monclús performed the seminal tests; S. García performed statistical analysis, M. Boada, A. Perez-Poch, M. Ballester, S. García-Monclús, and A.Veiga analyzed the data and wrote the paper. All authors read, reviewed, and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This work was approved by the Ethics Committee and Review Board of the Center.
Consent to participate
Study participants were informed of the procedure and gave their consent to participate by signing the informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Boada, M., Perez-Poch, A., Ballester, M. et al. Microgravity effects on frozen human sperm samples. J Assist Reprod Genet 37, 2249–2257 (2020). https://doi.org/10.1007/s10815-020-01877-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-020-01877-5