Abstract
Background
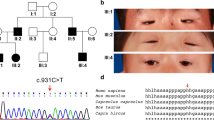
Blepharophimosis–ptosis–epicanthus inversus syndrome (BPES) is a rare, autosomal dominant disease. There are two clinical types of BPES: type I patients have eyelid abnormalities accompanied by infertility in affected females, while type II patients only display eyelid malformations. Previous studies have reported that the forkhead box L2 (FOXL2) gene mutations cause BPES.
Purpose
To identify plausible FOXL2 mutation in a Chinese family with BPES and infertility
Methods
Mutational screening of FOXL2 was performed in the affected members and 223 controls. Functional characterization of the novel mutation identified was carried out in vitro by luciferase reporter assay and subcellular localization experiment.
Results
A novel heterozygous mutation c.188 T > A (p.I63N) in FOXL2 was identified in two BPES patients in this family. The mutation abolished the transcriptional repression of FOXL2 on the promoters of CYP19A1 and CCND2 genes, as shown by luciferase reporter assays. However, no dominant-negative effect was observed for the mutation, and it did not impact FOXL2 protein nuclear localization and distribution.
Conclusions
The mutation c.188 T > A (p.I63N) in FOXL2 might be causative for BPES and infertility in this family and further amplified the spectrum of FOXL2 mutations.



Similar content being viewed by others
References
Bertini V, Valetto A, Baldinotti F, Azzarà A, Cambi F, Toschi B, et al. Blepharophimosis, ptosis, epicanthus inversus syndrome: new report with a 197-kb deletion upstream of FOXL2 and review of the literature. Molecular Syndromology. 2019;10:147–53.
Verdin H, De Baere E. Blepharophimosis, ptosis, and epicanthus inversus. University of Washington: GeneReviews; 2015. p. 1–18.
Zlotogora J, Sagi M, Cohen T. The blepharophimosis, ptosis, and epicanthus inversus syndrome: delineation of two types. Am J Hum Genet. 1983;35:1020–7.
Crisponi L, Deiana M, Loi A, Chiappe F, Uda M, Amati P, et al. The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat Genet. 2001;27:159–66.
Bunyan DJ, Thomas NS. Screening of a large cohort of blepharophimosis, ptosis, and epicanthus inversus syndrome patients reveals a very strong paternal inheritance bias and a wide spectrum of novel FOXL2 mutations. EUR J MED GENET. 2019;62:103668.
Verdin H, De Baere E. FOXL2 impairment in human disease. Horm Res Paediatr. 2012;77:2–11.
Cocquet J, Pailhoux E, Jaubert F, Servel N, Xia X, Pannetier M, et al. Evolution and expression of FOXL2. J Med Genet. 2002;39:916–21.
Uda M, Ottolenghi C, Crisponi L, Garcia JE, Deiana M, Kimber W, et al. Foxl2 disruption causes mouse ovarian failure by pervasive blockage of follicle development. Hum Mol Genet. 2004;13:1171–81.
Schmidt D, Ovitt CE, Anlag K, Fehsenfeld S, Gredsted L, Treier AC, et al. The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. DEVELOPMENT. 2004;131:933–42.
Bentsi-Barnes IK, Kuo FT, Barlow GM, Pisarska MD. Human forkhead L2 represses key genes in granulosa cell differentiation including aromatase, P450scc, and cyclin D2. Fertil Steril. 2010;94:353–6.
Kuo FT, Bentsi-Barnes IK, Barlow GM, Pisarska MD. Mutant Forkhead L2 (FOXL2) proteins associated with premature ovarian failure (POF) dimerize with wild-type FOXL2, leading to altered regulation of genes associated with granulosa cell differentiation. ENDOCRINOLOGY. 2011;152:3917–29.
Caburet S, Georges A, L'Hote D, Todeschini AL, Benayoun BA, Veitia RA. The transcription factor FOXL2: at the crossroads of ovarian physiology and pathology. Mol Cell Endocrinol. 2012;356:55–64.
Beysen D, De Paepe A, De Baere E. FOXL2 mutations and genomic rearrangements in BPES. Hum Mutat. 2009;30:158–69.
Fokstuen S, Antonarakis SE, Blouin JL. FOXL2-mutations in blepharophimosis-ptosis-epicanthus inversus syndrome (BPES); challenges for genetic counseling in female patients. Am J Med Genet A. 2003;117A:143–6.
De Baere E, Beysen D, Oley C, Lorenz B, Cocquet J, De Sutter P, et al. FOXL2 and BPES: mutational hotspots, phenotypic variability, and revision of the genotype-phenotype correlation. Am J Hum Genet. 2003;72:478–87.
De Baere E, Lemercier B, Christin-Maitre S, Durval D, Messiaen L, Fellous M, et al. FOXL2 mutation screening in a large panel of POF patients and XX males. J Med Genet. 2002;39:e43.
Dipietromaria A, Benayoun BA, Todeschini AL, Rivals I, Bazin C, Veitia RA. Towards a functional classification of pathogenic FOXL2 mutations using transactivation reporter systems. Hum Mol Genet. 2009;18:3324–33.
Meduri G, Bachelot A, Duflos C, Bstandig B, Poirot C, Genestie C, et al. FOXL2 mutations lead to different ovarian phenotypes in BPES patients: case report. Hum Reprod. 2010;25:235–43.
Elzaiat M, Todeschini AL, Caburet S, Veitia RA. The genetic make-up of ovarian development and function: the focus on the transcription factor FOXL2. Clin Genet. 2017;91:173–82.
Pisarska MD, Barlow G, Kuo FT. Minireview: roles of the forkhead transcription factor FOXL2 in granulosa cell biology and pathology. ENDOCRINOLOGY. 2011;152:1199–208.
Pisarska MD, Bae J, Klein C, Hsueh AJ. Forkhead l2 is expressed in the ovary and represses the promoter activity of the steroidogenic acute regulatory gene. ENDOCRINOLOGY. 2004;145:3424–33.
Park M, Suh DS, Lee K, Bae J. Positive cross talk between FOXL2 and antimullerian hormone regulates ovarian reserve. Fertil Steril. 2014;102:847–55.
Harris SE, Chand AL, Winship IM, Gersak K, Aittomaki K, Shelling AN. Identification of novel mutations in FOXL2 associated with premature ovarian failure. Mol Hum Reprod. 2002;8:729–33.
Laissue P, Lakhal B, Benayoun BA, Dipietromaria A, Braham R, Elghezal H, et al. Functional evidence implicating FOXL2 in non-syndromic premature ovarian failure and in the regulation of the transcription factor OSR2. J Med Genet. 2009;46:455–7.
Benayoun BA, Caburet S, Dipietromaria A, Bailly-Bechet M, Batista F, Fellous M, et al. The identification and characterization of a FOXL2 response element provides insights into the pathogenesis of mutant alleles. Hum Mol Genet. 2008;17:3118–27.
Beysen D, Moumné L, Veitia R, Peters H, Leroy BP, De Paepe A, et al. Missense mutations in the forkhead domain of FOXL2 lead to subcellular mislocalization, protein aggregation and impaired transactivation. Hum Mol Genet. 2008;17:2030–8.
Todeschini AL, Dipietromaria A, L'Hote D, Boucham FZ, Georges AB, Pandaranayaka PJ, et al. Mutational probing of the forkhead domain of the transcription factor FOXL2 provides insights into the pathogenicity of naturally occurring mutations. Hum Mol Genet. 2011;20:3376–85.
Saleem RA. Structural and functional analyses of disease-causing missense mutations in the forkhead domain of FOXC1. Hum Mol Genet. 2003;12:2993–3005.
Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation.
Georges A, L'Hote D, Todeschini AL, Auguste A, Legois B, Zider A, et al. The transcription factor FOXL2 mobilizes estrogen signaling to maintain the identity of ovarian granulosa cells. ELIFE. 2014;3.
Benayoun BA, Batista F, Auer J, Dipietromaria A, L'Hote D, De Baere E, et al. Positive and negative feedback regulates the transcription factor FOXL2 in response to cell stress: evidence for a regulatory imbalance induced by disease-causing mutations. Hum Mol Genet. 2009;18:632–44.
Benayoun BA, Georges AB, L'Hote D, Andersson N, Dipietromaria A, Todeschini AL, et al. Transcription factor FOXL2 protects granulosa cells from stress and delays cell cycle: role of its regulation by the SIRT1 deacetylase. Hum Mol Genet. 2011;20:1673–86.
Acknowledgments
The authors thank the patients for their participation. This work was supported by grants from the National Key Research & Developmental Program of China (2017YFC1001100), National Natural Science Foundation of China (81571505, 81571406 and 81873823), Shandong Medical and Health Science and Technology Development Project (2017WS741), Young Scholars Program of Shandong University (2016WLJH26), and the Fundamental Research Funds of Shandong University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Declaration
The authors have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hu, J., Ke, H., Luo, W. et al. A novel FOXL2 mutation in two infertile patients with blepharophimosis–ptosis–epicanthus inversus syndrome. J Assist Reprod Genet 37, 223–229 (2020). https://doi.org/10.1007/s10815-019-01651-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-019-01651-2